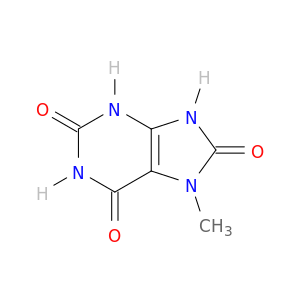
7-Methyl-1H-purine-2,6,8(3H,7H,9H)-trione
| Title | Journal |
|---|---|
| Methylated purines in urinary stones. | Clinical chemistry 20050801 |
| Uric acid may inhibit glucose-induced insulin secretion via binding to an essential arginine residue in rat pancreatic beta-cells. | Bioorganic & medicinal chemistry letters 20050215 |
| Catabolism of caffeine in plants and microorganisms. | Frontiers in bioscience : a journal and virtual library 20040501 |
| Structural basis for the binding affinity of xanthines with the DNA intercalator acridine orange. | Journal of medicinal chemistry 20011220 |
| Use of novel solid-phase extraction sorbent materials for high-performance liquid chromatography quantitation of caffeine metabolism products methylxanthines and methyluric acids in samples of biological origin. | Journal of chromatography. B, Biomedical sciences and applications 20010815 |