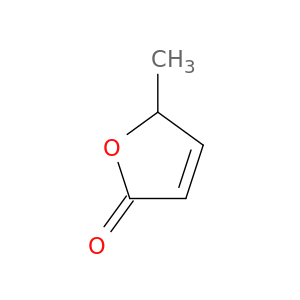
5-Methylfuran-2(5H)-one
| Title | Journal |
|---|---|
| Asymmetric assembly of 2-oxindole and α-angelica lactone units to construct vicinal quaternary chiral centers. | Chemical communications (Cambridge, England) 20120227 |
| Catalytic asymmetric vinylogous Mannich-type (AVM) reaction of nonactivated α-angelica lactone. | Organic letters 20110617 |
| Enhancement of glutathione and g-glutamylcysteine synthetase, the rate limiting enzyme of glutathione synthesis, by chemoprotective plant-derived food and beverage components in the human hepatoma cell line HepG2. | Nutrition and cancer 20030101 |
| Biosynthesis of acaterin: coupling of C(5) unit with octanoate. | The Journal of organic chemistry 20010824 |