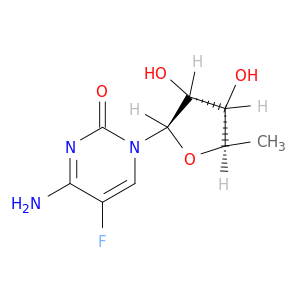
5'-Deoxy-5-fluorocytidine
| Title | Journal |
|---|---|
| Plasma disposition of capecitabine and its metabolites 5'DFCR and 5'DFUR in a standard and dose-intensified monotherapy regimen. | Cancer chemotherapy and pharmacology 20110301 |
| Plasma disposition of capecitabine (CCB) and its metabolites 5'-deoxy-5-fluorocytidine (5'-DFCR) and 5'-deoxy-5-fluorouracil (5'-DFUR) with two different capecitabine/oxaliplatin dosage regimens. | International journal of clinical pharmacology and therapeutics 20100701 |
| A new, validated HPLC-MS/MS method for the simultaneous determination of the anti-cancer agent capecitabine and its metabolites: 5'-deoxy-5-fluorocytidine, 5'-deoxy-5-fluorouridine, 5-fluorouracil and 5-fluorodihydrouracil, in human plasma. | Biomedical chromatography : BMC 20100401 |
| Compensatory effects of the human nucleoside transporters on the response to nucleoside-derived drugs in breast cancer MCF7 cells. | Biochemical pharmacology 20080201 |
| Gene expression predicts differential capecitabine metabolism, impacting on both pharmacokinetics and antitumour activity. | European journal of cancer (Oxford, England : 1990) 20080101 |
| A glucuronidation pathway of capecitabine occurs in rats but not in mice and humans. | Drug metabolism letters 20070401 |
| Rapid and simultaneous determination of capecitabine and its metabolites in mouse plasma, mouse serum, and in rabbit bile by high-performance liquid chromatography. | Journal of chromatography. A 20070105 |
| Simultaneous determination of capecitabine and its metabolites by HPLC and mass spectrometry for preclinical and clinical studies. | Journal of chromatography. B, Analytical technologies in the biomedical and life sciences 20051105 |
| Hydrolysis of capecitabine to 5'-deoxy-5-fluorocytidine by human carboxylesterases and inhibition by loperamide. | The Journal of pharmacology and experimental therapeutics 20050601 |
| Gene expression profiling revealed novel mechanism of action of Taxotere and Furtulon in prostate cancer cells. | BMC cancer 20050101 |
| Identification of the cytosolic carboxylesterase catalyzing the 5'-deoxy-5-fluorocytidine formation from capecitabine in human liver. | Drug metabolism and disposition: the biological fate of chemicals 20041001 |
| Bioactivation of capecitabine in human liver: involvement of the cytosolic enzyme on 5'-deoxy-5-fluorocytidine formation. | Drug metabolism and disposition: the biological fate of chemicals 20040701 |
| In vivo monitoring of capecitabine metabolism in human liver by 19fluorine magnetic resonance spectroscopy at 1.5 and 3 Tesla field strength. | Cancer research 20031115 |
| Isolation of an unknown metabolite of capecitabine, an oral 5-fluorouracil prodrug, and its identification by nuclear magnetic resonance and liquid chromatography-tandem mass spectrometry as a glucuroconjugate of 5'-deoxy-5-fluorocytidine, namely 2'-(beta-D-glucuronic acid)-5'-deoxy-5-fluorocytidine. | Journal of chromatography. B, Analytical technologies in the biomedical and life sciences 20030725 |
| Design and synthesis of the tumor-activated prodrug of dihydropyrimidine dehydrogenase (DPD) inhibitor, RO0094889 for combination therapy with capecitabine. | Bioorganic & medicinal chemistry letters 20030310 |
| Forced expression of cytidine deaminase confers sensitivity to capecitabine. | Oncology 20030101 |
| Penetration of capecitabine and its metabolites into malignant and healthy tissue of patients with advanced breast cancer. | International journal of clinical pharmacology and therapeutics 20021201 |