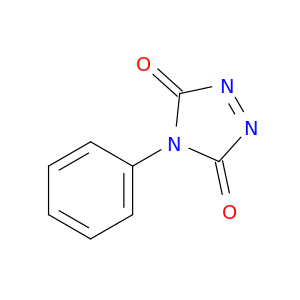
4-Phenyl-1,2,4-triazoline-3,5-dione
| Title | Journal |
|---|---|
| Gold catalysis: one-pot alkylideneoxazoline synthesis/Alder-ene reaction. | Chemistry, an Asian journal 20120601 |
| A rapid analytical method for cholecalciferol (vitamin D3) in fortified infant formula, milk and milk powder using Diels-Alder derivatisation and liquid chromatography-tandem mass spectrometric detection. | Analytical and bioanalytical chemistry 20120501 |
| Diels-Alder derivatization for sensitive detection and characterization of conjugated linoleic acids using LC/ESI-MS/MS. | Analytical and bioanalytical chemistry 20120401 |
| Advances in the synthesis of homochiral (-)-1-azafagomine and (+)-5-epi-1-azafagomine. 1-N-phenyl carboxamide derivatives of both enantiomers of 1-azafagomine: Leads for the synthesis of active α-glycosidase inhibitors. | The Journal of organic chemistry 20111202 |
| [4+2] cycloaddition reactions between 1,8-disubstituted cyclooctatetraenes and diazo dienophiles: stereoelectronic effects, anticancer properties and application to the synthesis of 7,8-substituted bicyclo[4.2.0]octa-2,4-dienes. | Chemistry (Weinheim an der Bergstrasse, Germany) 20100802 |
| Generation and intermolecular trapping of 1,2-diaza-4-silacyclopentane-3,5-diyls in the denitrogenation of 2,3,5,6-tetraaza-7-silabicyclo[2.2.1]hept-2-ene: an experimental and computational study. | The Journal of organic chemistry 20100319 |
| A sensitive LC/MS/MS assay of 25OH vitamin D3 and 25OH vitamin D2 in dried blood spots. | Clinica chimica acta; international journal of clinical chemistry 20090501 |
| A fluorous-tagged 'safety catch' linker for preparing heterocycles by ring-closing metathesis. | Organic letters 20090219 |
| Enzyme-catalysed synthesis and reactions of benzene oxide/oxepine derivatives of methyl benzoates. | Organic & biomolecular chemistry 20080407 |
| Oxa-ene reaction of enols of amides with 4-phenyl-1,2,4-triazoline-3,5-dione. | The Journal of organic chemistry 20080104 |
| Microsphere-based protease assays and screening application for lethal factor and factor Xa. | Cytometry. Part A : the journal of the International Society for Analytical Cytology 20060501 |
| Synthesis of 5-azacastanospermine, a conformationally restricted azafagomine analogue. | Chemistry (Weinheim an der Bergstrasse, Germany) 20010601 |
| Examination of structurally selective derivatization of vitamin D(3) analogues by electrospray mass spectrometry. | Journal of mass spectrometry : JMS 20010101 |