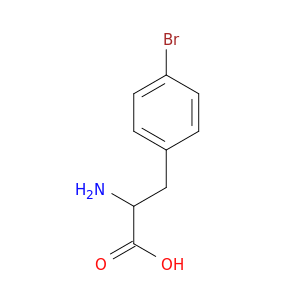
4-Bromo-L-Phenylalanine
| Title | Journal |
|---|---|
| Macrocyclic β-sheet peptides that inhibit the aggregation of a tau-protein-derived hexapeptide. | Journal of the American Chemical Society 20110309 |
| The de novo engineering of pyrrolysyl-tRNA synthetase for genetic incorporation of L-phenylalanine and its derivatives. | Molecular bioSystems 20110301 |
| X-ray crystallographic structure of an artificial beta-sheet dimer. | Journal of the American Chemical Society 20100825 |
| Site-specific incorporation of 4-iodo-L-phenylalanine through opal suppression. | Journal of biochemistry 20100801 |
| Design of a bacterial host for site-specific incorporation of p-bromophenylalanine into recombinant proteins. | Journal of the American Chemical Society 20060913 |
| Neighbouring group processes in the deamination of protonated phenylalanine derivatives. | Organic & biomolecular chemistry 20051021 |
| Asymmetric synthesis of N-protected amino acids by the addition of organolithium carboxyl synthons to ROPHy/SOPHy-derived aldoximes and ketoximes. | Organic & biomolecular chemistry 20040121 |
| Biphenyls as potent vitronectin receptor antagonists. Part 2: biphenylalanine ureas. | Bioorganic & medicinal chemistry letters 20030324 |
| Unnatural amino acid mutagenesis of green fluorescent protein. | The Journal of organic chemistry 20030110 |
| Influence of a ring substituent on the tendency to form H(2)O adducts to Ag(+) complexes with phenylalanine analogues in an ion trap mass spectrometer. | Journal of mass spectrometry : JMS 20020401 |