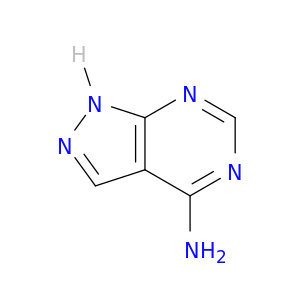
1H-Pyrazolo[3,4-d]pyrimidin-4-amine
| Title | Journal |
|---|---|
| Spectroscopic, electronic structure and natural bond analysis of 2-aminopyrimidine and 4-aminopyrazolo[3,4-d]pyrimidine: a comparative study. | Spectrochimica acta. Part A, Molecular and biomolecular spectroscopy 20121001 |
| Vibrational analysis of 4-amino pyrazolo (3,4-d) pyrimidine A joint FTIR, Laser Raman and scaled quantum mechanical studies. | Spectrochimica acta. Part A, Molecular and biomolecular spectroscopy 20111101 |
| Synthesis and characterization of two novel organic-inorganic compounds based on tetrahexyl and tetraheptyl ammonium ions and the Preyssler anion and their catalytic activities in the synthesis of 4-aminopyrazolo[3,4-d]- pyrimidines. | Molecules (Basel, Switzerland) 20100408 |
| Clinical grade production and characterization of a fusion protein comprised of the chemokine CCL2-ligand genetically fused to a mutated and truncated form of the Shiga A1 subunit. | Protein expression and purification 20090801 |
| The screening and characterization of 6-aminopurine-based xanthine oxidase inhibitors. | Bioorganic & medicinal chemistry 20070515 |
| Novel, highly potent adenosine deaminase inhibitors containing the pyrazolo[3,4-d]pyrimidine ring system. Synthesis, structure-activity relationships, and molecular modeling studies. | Journal of medicinal chemistry 20050811 |
| Prediction of genotoxicity of chemical compounds by statistical learning methods. | Chemical research in toxicology 20050601 |
| Synthesis and biological activity of 2'-deoxy-4'-thio-pyrazolo[3,4-d]pyrimidine nucleosides. | Nucleosides, nucleotides & nucleic acids 20050101 |
| [Riboflavin overproduction in 4-aminopyrazole[3,4-d]pyrimidine-treated yeast Pichia guilliermondii]. | Prikladnaia biokhimiia i mikrobiologiia 20020101 |
| Structure-activity relationships for the binding of ligands to xanthine or guanine phosphoribosyl-transferase from Toxoplasma gondii. | Biochemical pharmacology 19951109 |