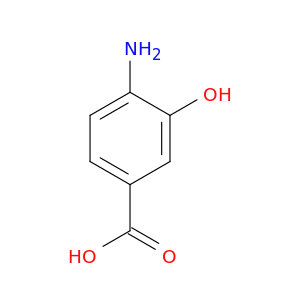
4-Amino-3-hydroxybenzoic acid
| Title | Journal |
|---|---|
| Redox potentials, laccase oxidation, and antilarval activities of substituted phenols. | Bioorganic & medicinal chemistry 20120301 |
| A copper-containing oxidase catalyzes C-nitrosation in nitrosobenzamide biosynthesis. | Nature chemical biology 20100901 |
| Gene cloning and characterization of a deaminase from the 4-amino-3-hydroxybenzoate-assimilating Bordetella sp. strain 10d. | FEMS microbiology letters 20090901 |
| 3D-QSAR and molecular docking studies of benzaldehyde thiosemicarbazone, benzaldehyde, benzoic acid, and their derivatives as phenoloxidase inhibitors. | Bioorganic & medicinal chemistry 20070301 |
| Metabolism of 4-amino-3-hydroxybenzoic acid by Bordetella sp. strain 10d: A different modified meta-cleavage pathway for 2-aminophenols. | Bioscience, biotechnology, and biochemistry 20061101 |
| Interaction of mushroom tyrosinase with aromatic amines, o-diamines and o-aminophenols. | Biochimica et biophysica acta 20040804 |
| A novel coupled enzyme assay reveals an enzyme responsible for the deamination of a chemically unstable intermediate in the metabolic pathway of 4-amino-3-hydroxybenzoic acid in Bordetella sp. strain 10d. | European journal of biochemistry 20040801 |
| Cloning of a gene encoding 4-amino-3-hydroxybenzoate 2,3-dioxygenase from Bordetella sp. 10d. | Biochemical and biophysical research communications 20040206 |
| A novel meta-cleavage dioxygenase that cleaves a carboxyl-group-substituted 2-aminophenol. Purification and characterization of 4-amino-3-hydroxybenzoate 2,3-dioxygenase from Bordetella sp. strain 10d. | European journal of biochemistry 20021201 |