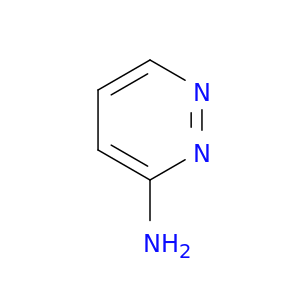
Pyridazin-3-amine
| Title | Journal |
|---|---|
| Discovery and Characterization of a Highly Potent and Selective Aminopyrazoline-Based in Vivo Probe (BAY-598) for the Protein Lysine Methyltransferase SMYD2. | Journal of medicinal chemistry 20160526 |
| Competitive antagonism of insect GABA receptors by iminopyridazine derivatives of GABA. | Bioorganic & medicinal chemistry 20121001 |
| In vivo evaluation of alpha7 nicotinic acetylcholine receptor agonists [11C]A-582941 and [11C]A-844606 in mice and conscious monkeys. | PloS one 20100101 |
| Polyfunctional nitriles in organic syntheses: a novel route to aminopyrroles, pyridazines and pyrazolo[3,4-c]pyridazines. | Molecules (Basel, Switzerland) 20090216 |
| A novel and convenient protocol for synthesis of pyridazines. | Organic letters 20090115 |
| R75761, a lead compound for the development of antiviral drugs in late stage poliomyelitis eradication strategies and beyond. | Antiviral research 20080601 |
| Privileged structures: a useful concept for the rational design of new lead drug candidates. | Mini reviews in medicinal chemistry 20071101 |
| Use of 4-bromo pyridazine 3,6-dione for building 3-amino pyridazine libraries. | Molecular diversity 20060801 |
| De novo and molecular target-independent discovery of orally bioavailable lead compounds for neurological disorders. | Current Alzheimer research 20060701 |
| Aminopyridazines attenuate hippocampus-dependent behavioral deficits induced by human beta-amyloid in a murine model of neuroinflammation. | Journal of molecular neuroscience : MN 20040101 |
| Aminopyridazines inhibit beta-amyloid-induced glial activation and neuronal damage in vivo. | Neurobiology of aging 20040101 |
| An aminopyridazine-based inhibitor of a pro-apoptotic protein kinase attenuates hypoxia-ischemia induced acute brain injury. | Bioorganic & medicinal chemistry letters 20031020 |
| Discovery of new chemical classes of synthetic ligands that suppress neuroinflammatory responses. | Journal of molecular neuroscience : MN 20020101 |
| Serotonin receptor and transporter ligands - current status. | Current medicinal chemistry 20010701 |
| Structure-based 3D QSAR and design of novel acetylcholinesterase inhibitors. | Journal of computer-aided molecular design 20010501 |