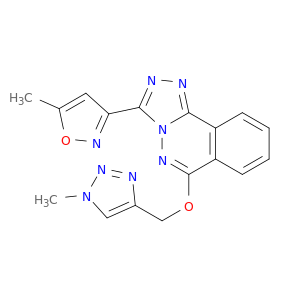
3-(5-Methylisoxazol-3-yl)-6-[(1-methyl-1H-1,2,3-triazol-4-yl)methoxy][1,2,4]triazolo[3,4-a]phthalazine
| Title | Journal |
|---|---|
| Specific targeting of the GABA-A receptor α5 subtype by a selective inverse agonist restores cognitive deficits in Down syndrome mice. | Journal of psychopharmacology (Oxford, England) 20110801 |
| Chronic Treatment with a Promnesiant GABA-A α5-Selective Inverse Agonist Increases Immediate Early Genes Expression during Memory Processing in Mice and Rectifies Their Expression Levels in a Down Syndrome Mouse Model. | Advances in pharmacological sciences 20110101 |
| Occupancy of human brain GABA(A) receptors by the novel α5 subtype-selective benzodiazepine site inverse agonist α5IA as measured using [¹¹C]flumazenil PET imaging. | Neuropharmacology 20101201 |
| The plasma-occupancy relationship of the novel GABAA receptor benzodiazepine site ligand, alpha5IA, is similar in rats and primates. | British journal of pharmacology 20090701 |
| An orally bioavailable, functionally selective inverse agonist at the benzodiazepine site of GABAA alpha5 receptors with cognition enhancing properties. | Journal of medicinal chemistry 20041118 |