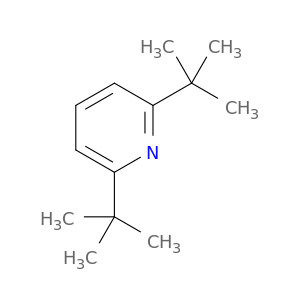
2,6-Di-tert-butylpyridine
| Title | Journal |
|---|---|
| Silylium ion-catalyzed challenging Diels-Alder reactions: the danger of hidden proton catalysis with strong Lewis acids. | Journal of the American Chemical Society 20120307 |
| Letter: A simple ion source set-up for desorption/ionization on silicon with ion mobility spectrometry and ion mobility spectrometry-mass spectrometry. | European journal of mass spectrometry (Chichester, England) 20110101 |
| MSU-S mesoporous materials: an efficient catalyst for isomerization of alpha-pinene. | Bioresource technology 20101001 |
| Separation of different ion structures in atmospheric pressure photoionization-ion mobility spectrometry-mass spectrometry (APPI-IMS-MS). | Journal of the American Society for Mass Spectrometry 20100901 |
| Consequences of acid strength for isomerization and elimination catalysis on solid acids. | Journal of the American Chemical Society 20090513 |
| Adjusting mobility scales of ion mobility spectrometers using 2,6-DtBP as a reference compound. | Talanta 20080915 |
| Interfacing an aspiration ion mobility spectrometer to a triple quadrupole mass spectrometer. | The Review of scientific instruments 20070401 |
| Intrinsic acidity of dimethylhalonium ions: evidence for hyperconjugation in dimethylhalonium ylides in the gas phase. | The Journal of organic chemistry 20060331 |
| Probing surface basicity of solid acids with an aminobenzodifurandione dye as the solvatochromic probe. | The journal of physical chemistry. B 20050421 |
| Tetraalkylammonium halides as chemical standards for positive electrospray ionization with ion mobility spectrometry/mass spectrometry. | Rapid communications in mass spectrometry : RCM 20050101 |
| Simple synthesis of a weak nucleophilic base (4-ethyl-2,6-diisopropyl-3,5-dimethylpyridine) evidencing a double Janus group effect. | The Journal of organic chemistry 20040123 |
| Evidence that protons can be the active catalysts in Lewis acid mediated hetero-Michael addition reactions. | Chemistry (Weinheim an der Bergstrasse, Germany) 20040123 |
| Development of an ion mobility spectrometer for use in an atmospheric pressure ionization ion mobility spectrometer/mass spectrometer instrument for fast screening analysis. | Rapid communications in mass spectrometry : RCM 20040101 |
| Tuning the strain and polymerizability of organometallic rings: the synthesis, structure, and ring-opening polymerization behavior of [2]ferrocenophanes with C-SI, C-P, and C-S bridges. | Journal of the American Chemical Society 20010314 |