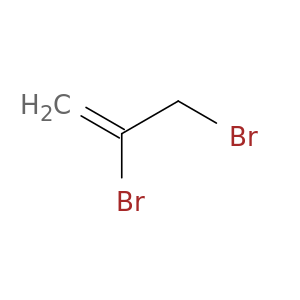
2,3-Dibromopropene, tech grade
| Title | Journal |
|---|---|
| Formal synthesis of (+/-)-platensimycin. | Organic letters 20070426 |
| Identification of glutathione conjugates of 2, 3-dibromopropene in male ICR mice. | Archives of pharmacal research 20060201 |
| Novel carbonyl bromoallylation/Heck reaction sequence. Stereocontrolled access to bicyclic beta-lactams. | The Journal of organic chemistry 20050401 |
| Straightforward asymmetric entry to highly functionalized 3-substituted 3-hydroxy-beta-lactams via Baylis-Hillman or bromoallylation reactions. | The Journal of organic chemistry 20040206 |
| Haloalkane dehalogenase LinB from Sphingomonas paucimobilis UT26: X-ray crystallographic studies of dehalogenation of brominated substrates. | Biochemistry 20030902 |
| Controlling chemoselectivity in vinyl and allylic C-X bond activation with palladium catalysis: a pK(a)-based electronic switch. | Journal of the American Chemical Society 20020220 |