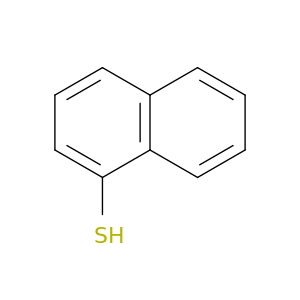
Naphthalene-1-thiol
| Title | Journal |
|---|---|
| Shedding light into the detailed excited-state relaxation pathways and reaction mechanisms of thionaphthol isomers. | The journal of physical chemistry. A 20110210 |
| SERS-active gold lace nanoshells with built-in hotspots. | Nano letters 20101013 |
| Pattern-based recognition of thiols and metals using a single squaraine indicator. | Journal of the American Chemical Society 20090916 |
| Site-specific radical directed dissociation of peptides at phosphorylated residues. | Journal of the American Chemical Society 20080917 |
| S-S bond mesolysis in alpha,alpha'-dinaphthyl disulfide radical anion generated during gamma-radiolysis and pulse radiolysis in organic solution. | The journal of physical chemistry. A 20061221 |
| Non-nucleoside HIV reverse transcriptase inhibitors, Part 6[1]: synthesis and anti-HIV activity of novel 2-[(arylcarbonylmethyl)thio]-6-arylthio DABO analogues. | Archiv der Pharmazie 20051001 |
| 1,2-Thio group migration in Rh(II) carbene reactions. | The Journal of organic chemistry 20050513 |
| Surface-enhanced Raman scattering for ultrasensitive chemical analysis of 1 and 2-naphthalenethiols. | The Analyst 20041201 |
| New paeonilactone-A adducts formed by anaerobic incubation of paeoniflorin with Lactobacillus brevis in the presence of arylthiols. | Chemical & pharmaceutical bulletin 20010701 |