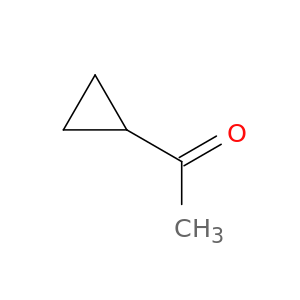
Cyclopropyl methyl ketone
| Title | Journal |
|---|---|
| Male-produced sex pheromone of the stink bug Edessa meditabunda. | Journal of chemical ecology 20120701 |
| Preparation and evaluation of 5, 9-dimethyl-2-cyclopropyl-2-decanol as a penetration enhancer for drugs through rat skin. | Pakistan journal of pharmaceutical sciences 20111001 |
| Synthesis and reactivity of six-membered oxa-nickelacycles: a ring-opening reaction of cyclopropyl ketones. | Chemistry (Weinheim an der Bergstrasse, Germany) 20091005 |
| (Z)- and (E)-2-(1,2-dihydroxyethyl)methylenecyclopropane analogues of 2'-deoxyadenosine and 2'-deoxyguanosine. Synthesis of all stereoisomers, absolute configuration, and antiviral activity. | Journal of medicinal chemistry 20090528 |
| Synthesis of spiropyrans: H-abstractions in 3-cycloalkenyloxybenzopyrans. | Beilstein journal of organic chemistry 20070101 |
| Synthesis of 1,2,4-trioxepanes and 1,2,4-trioxocanes via photooxygenation of homoallylic alcohols. | The Journal of organic chemistry 20061124 |
| Designing the 'search pathway' in the development of a new class of highly efficient stereoselective hydrosilylation catalysts. | Chemistry (Weinheim an der Bergstrasse, Germany) 20050422 |