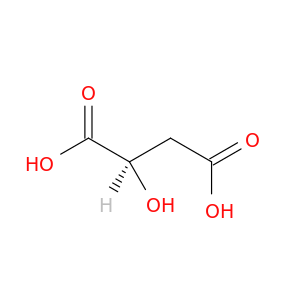
D-(+)-Malic acid
| Title | Journal |
|---|---|
| AcsD catalyzes enantioselective citrate desymmetrization in siderophore biosynthesis. | Nature chemical biology 20090301 |
| Total synthesis of leustroducsin B. | The Journal of organic chemistry 20080718 |
| Substrate specificity analysis and inhibitor design of homoisocitrate dehydrogenase. | Bioorganic & medicinal chemistry 20070201 |
| Microsphere-based protease assays and screening application for lethal factor and factor Xa. | Cytometry. Part A : the journal of the International Society for Analytical Cytology 20060501 |
| Diversity of function in the isocitrate lyase enzyme superfamily: the Dianthus caryophyllus petal death protein cleaves alpha-keto and alpha-hydroxycarboxylic acids. | Biochemistry 20051220 |
| A total synthesis of hydroxylysine in protected form and investigations of the reductive opening of p-methoxybenzylidene acetals. | The Journal of organic chemistry 20041210 |
| Total synthesis of an antitumor antibiotic, Fostriecin (CI-920). | Journal of the American Chemical Society 20030709 |
| Total synthesis and structure confirmation of the annonaceous acetogenins 30(S)-hydroxybullatacin, uvarigrandin a, and 5(R)-uvarigrandin a (narumicin I?). | The Journal of organic chemistry 20030307 |