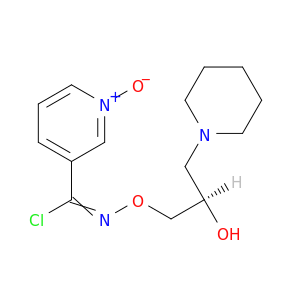
3-Pyridinecarboximidoyl chloride, N-[(2R)-2-hydroxy-3-(1-piperidinyl)propoxy]-, 1-oxide
| Title | Journal |
|---|---|
| Treatment with a coinducer of the heat shock response delays muscle denervation in the SOD1-G93A mouse model of amyotrophic lateral sclerosis. | Amyotrophic lateral sclerosis : official publication of the World Federation of Neurology Research Group on Motor Neuron Diseases 20120601 |
| Plasma neurofilament heavy chain levels correlate to markers of late stage disease progression and treatment response in SOD1(G93A) mice that model ALS. | PloS one 20120101 |
| Arimoclomol, a coinducer of heat shock proteins for the potential treatment of amyotrophic lateral sclerosis. | IDrugs : the investigational drugs journal 20100701 |
| Arimoclomol: a potential therapy under development for ALS. | Expert opinion on investigational drugs 20091201 |
| Activation of the heat shock response in a primary cellular model of motoneuron neurodegeneration-evidence for neuroprotective and neurotoxic effects. | Cellular & molecular biology letters 20090101 |
| Late stage treatment with arimoclomol delays disease progression and prevents protein aggregation in the SOD1 mouse model of ALS. | Journal of neurochemistry 20081001 |
| Arimoclomol at dosages up to 300 mg/day is well tolerated and safe in amyotrophic lateral sclerosis. | Muscle & nerve 20080701 |
| Treatment with arimoclomol, a coinducer of heat shock proteins, delays disease progression in ALS mice. | Nature medicine 20040401 |
| Putting the heat on ALS. | Nature medicine 20040401 |
| The effect of treatment with BRX-220, a co-inducer of heat shock proteins, on sensory fibers of the rat following peripheral nerve injury. | Experimental neurology 20031201 |
| Upregulation of heat shock proteins rescues motoneurones from axotomy-induced cell death in neonatal rats. | Experimental neurology 20020701 |
| Nontoxic heat shock protein coinducer BRX-220 protects against acute pancreatitis in rats. | Free radical biology & medicine 20020615 |
| Comparison of the extrapancreatic action of BRX-220 and pioglitazone in the high-fat diet-induced insulin resistance. | Annals of the New York Academy of Sciences 20020601 |
| Effect of BRX-220 against peripheral neuropathy and insulin resistance in diabetic rat models. | Annals of the New York Academy of Sciences 20020601 |