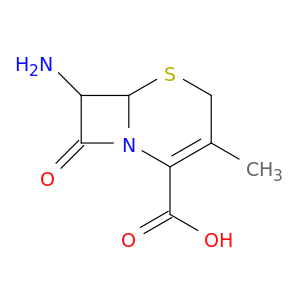
5-Thia-1-azabicyclo[4.2.0]oct-2-ene-2-carboxylic acid, 7-amino-3-methyl-8-oxo-
| Title | Journal |
|---|---|
| Anaerobic stabilization and conversion of transformed intermediates of antibiotic pharmaceutical effluent in a fluidized bed reactor. | Journal of environmental science & engineering 20110701 |
| Effect of loading rate and HRT on the removal of cephalosporin and their intermediates during the operation of a membrane bioreactor treating pharmaceutical wastewater. | Environmental technology 20090901 |
| Enzymatic preparation of cefaclor with immobilized penicillin acylase. | Preparative biochemistry & biotechnology 20080101 |
| Penicillin acylase-catalyzed synthesis of beta-lactam antibiotics in highly condensed aqueous systems: beneficial impact of kinetic substrate supersaturation. | Biotechnology and bioengineering 20040205 |
| Analysis of a substrate specificity switch residue of cephalosporin acylase. | Biochemical and biophysical research communications 20031219 |
| Kinetics of enzyme acylation and deacylation in the penicillin acylase-catalyzed synthesis of beta-lactam antibiotics. | European journal of biochemistry 20030901 |
| Continuous cultivations of a Penicillium chrysogenum strain expressing the expandase gene from Streptomyces clavuligerus: Growth yields and morphological characterization. | Biotechnology and bioengineering 20030805 |
| Enhanced enzymatic synthesis of a semi-synthetic cephalosprin, cefaclor, with in situ product removal. | Biotechnology letters 20030701 |
| Integrated reactor concepts for the enzymatic kinetic synthesis of cephalexin. | Biotechnology and bioengineering 20021020 |
| Quantitative characterization of the nucleophile reactivity in penicillin acylase-catalyzed acyl transfer reactions. | Biochimica et biophysica acta 20020923 |
| Advantages of using non-isothermal bioreactors for the enzymatic synthesis of antibiotics: the penicillin G acylase as enzyme model. | Biotechnology and bioengineering 20020805 |
| A two-step, one-pot enzymatic synthesis of cephalexin from D-phenylglycine nitrile. | Biotechnology and bioengineering 20020805 |
| Enzyme reaction engineering: effect of methanol on the synthesis of antibiotics catalyzed by immobilized penicillin G acylase under isothermal and non-isothermal conditions. | Biotechnology progress 20020101 |
| Modeling of the enzymatic kinetic synthesis of cephalexin--influence of substrate concentration and temperature. | Biotechnology and bioengineering 20010505 |
| Probing the penicillin sidechain selectivity of recombinant deacetoxycephalosporin C synthase. | Cellular and molecular life sciences : CMLS 20010501 |