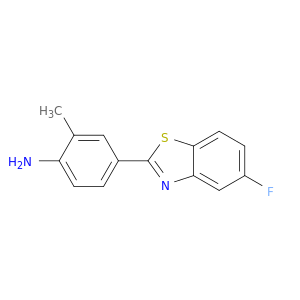
Benzenamine, 4-(5-fluoro-2-benzothiazolyl)-2-methyl-
| Title | Journal |
|---|---|
| Aryl Hydrocarbon Receptor Ligand 5F 203 Induces Oxidative Stress That Triggers DNA Damage in Human Breast Cancer Cells. | Chemical research in toxicology 20150518 |
| Reduction of aromatic and heterocyclic aromatic N-hydroxylamines by human cytochrome P450 2S1. | Chemical research in toxicology 20130617 |
| Synthesis and biological evaluation of novel triazoles and isoxazoles linked 2-phenyl benzothiazole as potential anticancer agents. | Bioorganic & medicinal chemistry letters 20120901 |
| Bioactivation of fluorinated 2-aryl-benzothiazole antitumor molecules by human cytochrome P450s 1A1 and 2W1 and deactivation by cytochrome P450 2S1. | Chemical research in toxicology 20120820 |
| CYP2S1 and CYP2W1 mediate 2-(3,4-dimethoxyphenyl)-5-fluorobenzothiazole (GW-610, NSC 721648) sensitivity in breast and colorectal cancer cells. | Molecular cancer therapeutics 20111001 |
| Variations in Mre11/Rad50/Nbs1 status and DNA damage-induced S-phase arrest in the cell lines of the NCI60 panel. | BMC cancer 20110101 |
| The role of aryl hydrocarbon receptor and crosstalk with estrogen receptor in response of breast cancer cells to the novel antitumor agents benzothiazoles and aminoflavone. | International journal of breast cancer 20110101 |
| 2-(4-Amino-3-methylphenyl)-5-fluorobenzothiazole is a ligand and shows species-specific partial agonism of the aryl hydrocarbon receptor. | Toxicology and applied pharmacology 20090515 |
| Preclinical toxicokinetic evaluation of phortress [2-(4-amino-3-methylphenyl)-5-fluorobenzothiazole lysylamide dihydrochloride] in two rodent species. | Pharmacology 20090101 |
| Cytochrome P450 CYP1A1: wider roles in cancer progression and prevention. | BMC cancer 20090101 |
| BRCA1 transcriptional activity is enhanced by interactions between its AD1 domain and AhR. | Cancer chemotherapy and pharmacology 20081101 |
| BRCA1 modulates sensitivity to 5F-203 by regulating xenobiotic stress-inducible protein levels and EROD activity. | Cancer chemotherapy and pharmacology 20080901 |
| Mechanisms of acquired resistance to 2-(4-Amino-3-methylphenyl)benzothiazole in breast cancer cell lines. | Breast cancer research and treatment 20080701 |
| Drug-induced expression of nonsteroidal anti-inflammatory drug-activated gene/macrophage inhibitory cytokine-1/prostate-derived factor, a putative tumor suppressor, inhibits tumor growth. | The Journal of pharmacology and experimental therapeutics 20060801 |
| Anti-tumor drug candidate 2-(4-amino-3-methylphenyl)-5-fluorobenzothiazole induces single-strand breaks and DNA-protein cross-links in sensitive MCF-7 breast cancer cells. | Cancer chemotherapy and pharmacology 20060701 |
| Structural studies on bioactive compounds. 40.(1) Synthesis and biological properties of fluoro-, methoxyl-, and amino-substituted 3-phenyl-4H-1-benzopyran-4-ones and a comparison of their antitumor activities with the activities of related 2-phenylbenzothiazoles. | Journal of medicinal chemistry 20060629 |
| BRCA1 modulates xenobiotic stress-inducible gene expression by interacting with ARNT in human breast cancer cells. | The Journal of biological chemistry 20060526 |
| Microsphere-based protease assays and screening application for lethal factor and factor Xa. | Cytometry. Part A : the journal of the International Society for Analytical Cytology 20060501 |
| Differential gene expression as a potential classifier of 2-(4-amino-3-methylphenyl)-5-fluorobenzothiazole-sensitive and -insensitive cell lines. | Molecular pharmacology 20060301 |
| Antitumor benzothiazoles. 26.(1) 2-(3,4-dimethoxyphenyl)-5-fluorobenzothiazole (GW 610, NSC 721648), a simple fluorinated 2-arylbenzothiazole, shows potent and selective inhibitory activity against lung, colon, and breast cancer cell lines. | Journal of medicinal chemistry 20060112 |
| The antitumor drug candidate 2-(4-amino-3-methylphenyl)-5-fluorobenzothiazole induces NF-kappaB activity in drug-sensitive MCF-7 cells. | Anti-cancer drugs 20050201 |
| Fluorinated 2-(4-amino-3-methylphenyl)benzothiazoles induce CYP1A1 expression, become metabolized, and bind to macromolecules in sensitive human cancer cells. | Drug metabolism and disposition: the biological fate of chemicals 20041201 |
| In vitro, in vivo, and in silico analyses of the antitumor activity of 2-(4-amino-3-methylphenyl)-5-fluorobenzothiazoles. | Molecular cancer therapeutics 20041201 |
| The experimental antitumor agents Phortress and doxorubicin are equiactive against human-derived breast carcinoma xenograft models. | Breast cancer research and treatment 20040901 |
| The development of the antitumour benzothiazole prodrug, Phortress, as a clinical candidate. | Current medicinal chemistry 20040401 |
| Induction of CYP1A1 in tumor cells by the antitumor agent 2-[4-amino-3-methylphenyl]-5-fluoro-benzothiazole: a potential surrogate marker for patient sensitivity. | Molecular cancer therapeutics 20031201 |
| Genotoxic profiling of MCF-7 breast cancer cell line elucidates gene expression modifications underlying toxicity of the anticancer drug 2-(4-amino-3-methylphenyl)-5-fluorobenzothiazole. | Molecular pharmacology 20030301 |
| DNA damage and cell cycle arrest induced by 2-(4-amino-3-methylphenyl)-5-fluorobenzothiazole (5F 203, NSC 703786) is attenuated in aryl hydrocarbon receptor deficient MCF-7 cells. | British journal of cancer 20030224 |
| Antitumour 2-(4-aminophenyl)benzothiazoles generate DNA adducts in sensitive tumour cells in vitro and in vivo. | British journal of cancer 20030210 |
| In vitro evaluation of amino acid prodrugs of novel antitumour 2-(4-amino-3-methylphenyl)benzothiazoles. | British journal of cancer 20020422 |
| Preclinical evaluation of amino acid prodrugs of novel antitumor 2-(4-amino-3-methylphenyl)benzothiazoles. | Molecular cancer therapeutics 20020201 |
| Antitumor benzothiazoles. 16. Synthesis and pharmaceutical properties of antitumor 2-(4-aminophenyl)benzothiazole amino acid prodrugs. | Journal of medicinal chemistry 20020131 |
| Antitumor benzothiazoles. 14. Synthesis and in vitro biological properties of fluorinated 2-(4-aminophenyl)benzothiazoles. | Journal of medicinal chemistry 20010426 |