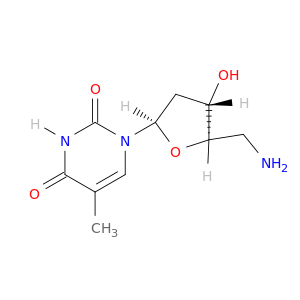
Thymidine, 5'-amino-5'-deoxy-
| Title | Journal |
|---|---|
| Kinetic parameters and recognition of thymidine analogues with varying functional groups by thymidine phosphorylase. | Bioorganic & medicinal chemistry 20080401 |
| Rational design of 5'-thiourea-substituted alpha-thymidine analogues as thymidine monophosphate kinase inhibitors capable of inhibiting mycobacterial growth. | Journal of medicinal chemistry 20071101 |
| 3D-QSAR studies on antitubercular thymidine monophosphate kinase inhibitors based on different alignment methods. | Bioorganic & medicinal chemistry letters 20060215 |
| Thymidine and thymidine-5'-O-monophosphate analogues as inhibitors of Mycobacterium tuberculosis thymidylate kinase. | Bioorganic & medicinal chemistry letters 20030915 |
| X-irradiation effects on thymidine kinase (TK): II. The significance of deoxythymidine triphosphate for inhibition of TK1 activity. | Cell proliferation 20020401 |
| Structure-activity relationships of pyrimidine nucleosides as antiviral agents for human immunodeficiency virus type 1 in peripheral blood mononuclear cells. | Journal of medicinal chemistry 19890301 |
| Synthesis and antiviral activity of various 3'-azido, 3'-amino, 2',3'-unsaturated, and 2',3'-dideoxy analogues of pyrimidine deoxyribonucleosides against retroviruses. | Journal of medicinal chemistry 19870201 |
| Nucleosides. 139. Synthesis and anticytomegalovirus and antiherpes simplex virus activity of 5'-modified analogues of 2'-fluoroarabinosylpyrimidine nucleosides. | Journal of medicinal chemistry 19870101 |
| Synthesis of some 5'-amino-2',5'-dideoxy-5-iodouridine derivatives and their antiviral properties against herpes simplex virus. | Antiviral research 19821201 |
| Synthesis and antiviral activity of 5- and 5'-substituted thymidine analogs. | Journal of medicinal chemistry 19760401 |