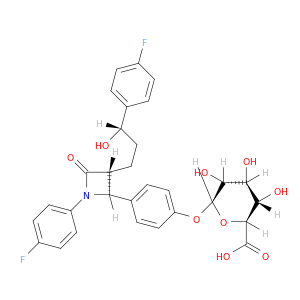
β-D-Glucopyranosiduronic acid, 4-[(2S,3R)-1-(4-fluorophenyl)-3-[(3S)-3-(4-fluorophenyl)-3-hydroxypropyl]-4-oxo-2-azetidinyl]phenyl
| Title | Journal |
|---|---|
| Molecular mechanism of altered ezetimibe disposition in nonalcoholic steatohepatitis. | Drug metabolism and disposition: the biological fate of chemicals 20120301 |
| Substituted oxazolidinones as novel NPC1L1 ligands for the inhibition of cholesterol absorption. | Bioorganic & medicinal chemistry letters 20080115 |
| Disposition of the selective cholesterol absorption inhibitor ezetimibe in healthy male subjects. | Drug metabolism and disposition: the biological fate of chemicals 20020401 |
| Synthesis of fluorescent biochemical tools related to the 2-azetidinone class of cholesterol absorption inhibitors. | Bioorganic & medicinal chemistry letters 20020211 |