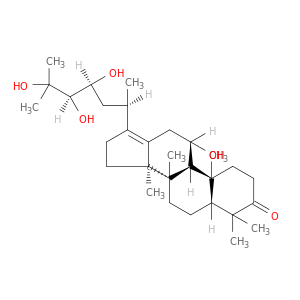
Dammar-13(17)-en-3-one, 11,23,24,25-tetrahydroxy-, (8α,9β,11β,14β,23S,24R)-
| Title | Journal |
|---|---|
| Renaissance remedies: Antiplasmodial protostane triterpenoids from Alisma plantago-aquatica L. (Alismataceae). | Journal of ethnopharmacology 20110426 |
| A sensitive liquid chromatography-mass spectrometry method for simultaneous determination of alisol A and alisol A 24-acetate from Alisma orientale (Sam.) Juz. in rat plasma. | Analytical and bioanalytical chemistry 20110101 |
| Effects of protostane-type triterpenoids on the 5-HT3A receptor-mediated ion current in Xenopus oocytes. | Brain research 20100517 |
| Anti-HBV agents. Part 3: preliminary structure-activity relationships of tetra-acylalisol A derivatives as potent hepatitis B virus inhibitors. | Bioorganic & medicinal chemistry letters 20091201 |
| Anti-HBV agents. Part 2: synthesis and in vitro anti-hepatitis B virus activities of alisol A derivatives. | Bioorganic & medicinal chemistry letters 20090415 |
| [Chemical constituents of Alisma orientalis and their immunosuppressive function]. | Zhongguo Zhong yao za zhi = Zhongguo zhongyao zazhi = China journal of Chinese materia medica 20090401 |
| Effects of sesquiterpenes and triterpenes from the rhizome of Alisma orientale on nitric oxide production in lipopolysaccharide-activated macrophages: absolute stereostructures of alismaketones-B 23-acetate and -C 23-acetate. | Bioorganic & medicinal chemistry letters 19991101 |