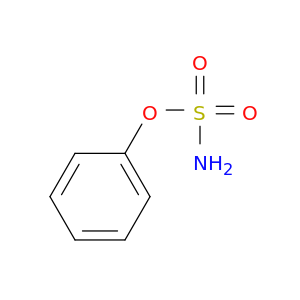
Sulfamic acid, phenyl ester
| Title | Journal |
|---|---|
| Efficacious N-protection of O-aryl sulfamates with 2,4-dimethoxybenzyl groups. | Organic & biomolecular chemistry 20121007 |
| Molecular cloning, characterization, and inhibition studies of a β-carbonic anhydrase from Malassezia globosa, a potential antidandruff target. | Journal of medicinal chemistry 20120412 |
| Aromatase and dual aromatase-steroid sulfatase inhibitors from the letrozole and vorozole templates. | ChemMedChem 20110801 |
| Carbonic anhydrase inhibitors; fluorinated phenyl sulfamates show strong inhibitory activity and selectivity for the inhibition of the tumor-associated isozymes IX and XII over the cytosolic ones I and II. | Bioorganic & medicinal chemistry letters 20090901 |
| Recent developments of steroid sulfatase inhibitors as anti-cancer agents. | Anti-cancer agents in medicinal chemistry 20081001 |
| Dual aromatase-sulfatase inhibitors based on the anastrozole template: synthesis, in vitro SAR, molecular modelling and in vivo activity. | Organic & biomolecular chemistry 20071021 |
| Dual aromatase-sulfatase inhibitors based on the anastrozole template: synthesis, in vitro SAR, molecular modelling and in vivo activity. | Organic & biomolecular chemistry 20070921 |
| Synthesis and evaluation of general mechanism-based inhibitors of sulfatases based on (difluoro)methyl phenyl sulfate and cyclic phenyl sulfamate motifs. | Bioorganic & medicinal chemistry 20061215 |
| Microsphere-based protease assays and screening application for lethal factor and factor Xa. | Cytometry. Part A : the journal of the International Society for Analytical Cytology 20060501 |
| Carbonic anhydrase inhibitors: inhibition of the human isozymes I, II, VA, and IX with a library of substituted difluoromethanesulfonamides. | Bioorganic & medicinal chemistry letters 20051201 |
| Steroid sulfatase: molecular biology, regulation, and inhibition. | Endocrine reviews 20050401 |
| A letrozole-based dual aromatase-sulphatase inhibitor with in vivo activity. | The Journal of steroid biochemistry and molecular biology 20050201 |
| Carbonic anhydrase inhibitors. Inhibition of cytosolic isozymes I and II and transmembrane, tumor-associated isozyme IX with sulfamates including EMATE also acting as steroid sulfatase inhibitors. | Journal of medicinal chemistry 20030522 |
| Structure-activity relationship determination within a group of substituted phenyl sulfamate based compounds against the enzyme oestrone sulfatase. | The Journal of pharmacy and pharmacology 20030201 |
| The mechanism of the irreversible inhibition of estrone sulfatase (ES) through the consideration of a range of methane- and amino-sulfonate-based compounds. | Bioorganic & medicinal chemistry letters 20020506 |
| Acid dissociation constant, a potential physicochemical factor in the inhibition of the enzyme estrone sulfatase (ES). | Bioorganic & medicinal chemistry letters 20010409 |