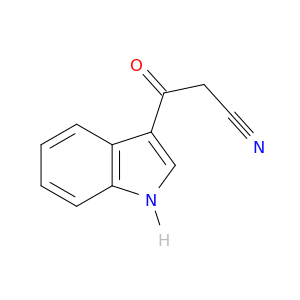
3-(1H-Indol-3-yl)-3-oxopropanenitrile
| Title | Journal |
|---|---|
| 4-(4-Chloro-phen-yl)-2,6-bis-(1H-indol-3-yl)-1,4-dihydro-pyridine-3,5-dicarbo-nitrile ethanol monosolvate. | Acta crystallographica. Section E, Structure reports online 20120501 |
| Synthesis and SAR studies of 1,4-benzoxazine MenB inhibitors: novel antibacterial agents against Mycobacterium tuberculosis. | Bioorganic & medicinal chemistry letters 20101101 |
| InCl3 mediated one-pot multicomponent synthesis, anti-microbial, antioxidant and anticancer evaluation of 3-pyranyl indole derivatives. | Bioorganic & medicinal chemistry letters 20100901 |
| Simple and convenient approach to the Kreohnke pyridine type synthesis of functionalized indol-3-yl pyridine derivatives using 3-cyanoacetyl indole. | Journal of combinatorial chemistry 20100101 |
| 6-(1H-Indol-3-yl)-4-phenyl-2,2'-bi-pyridine-5-carbonitrile. | Acta crystallographica. Section E, Structure reports online 20090601 |
| 2-(1H-indol-3-ylcarbon-yl)acetonitrile. | Acta crystallographica. Section E, Structure reports online 20090301 |
| 4-(4-Bromo-phen-yl)-6-(1H-indol-3-yl)-2,2'-bipyridine-5-carbonitrile. | Acta crystallographica. Section E, Structure reports online 20090301 |
| 4-(2,4-Dichlorophenyl)-2-(1H-indol-3-yl)-6-(2-pyridyl)-1,4-dihydropyridine-4-carbonitrile. | Acta crystallographica. Section E, Structure reports online 20081001 |
| 4-(2,4-Dichlorophenyl)-2-(1H-indol-3-yl)-6-methoxypyridine-3,5-dicarbonitrile. | Acta crystallographica. Section E, Structure reports online 20081001 |
| Synthesis and biological evaluation of new 3-substituted indole derivatives as potential anti-inflammatory and analgesic agents. | Bioorganic & medicinal chemistry 20070601 |
| Synthesis and antitumor activity of indolylpyrimidines: marine natural product meridianin D analogues. | Bioorganic & medicinal chemistry 20070201 |