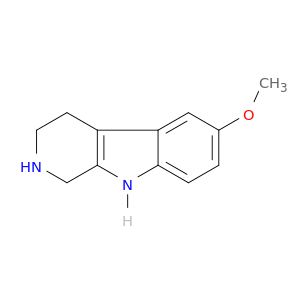
6-Methoxy-2,3,4,9-tetrahydro-1h-pyrido[3,4-b]indole
| Title | Journal |
|---|---|
| Prospective acetylcholinesterase inhibitory activity of indole and its analogs. | Bioorganic & medicinal chemistry letters 20120415 |
| Effects of spider venom toxin PWTX-I (6-Hydroxytrypargine) on the central nervous system of rats. | Toxins 20110201 |
| In vivo hepatic oxidative stress because of carbon tetrachloride toxicity: protection by melatonin and pinoline. | Journal of pineal research 20100801 |
| Melatonin and structurally-related compounds protect synaptosomal membranes from free radical damage. | International journal of molecular sciences 20100101 |
| Pinoline may be used as a probe for CYP2D6 activity. | Drug metabolism and disposition: the biological fate of chemicals 20090301 |
| Pinoline and N-acetylserotonin reduce glutamate-induced lipid peroxidation in retinal homogenates. | Neuroscience letters 20070202 |
| 6-Hydroxy- and 6-methoxy-beta-carbolines as acetyl- and butyrylcholinesterase inhibitors. | Bioorganic & medicinal chemistry letters 20061115 |
| Protective effect of melatonin and pinoline on nitric oxide-induced lipid and protein peroxidation in rat brain homogenates. | Neuroscience letters 20060911 |
| Microsphere-based protease assays and screening application for lethal factor and factor Xa. | Cytometry. Part A : the journal of the International Society for Analytical Cytology 20060501 |
| Antioxidant activity of melatonin and a pinoline derivative on linoleate model system. | Journal of pineal research 20050801 |
| Prediction of genotoxicity of chemical compounds by statistical learning methods. | Chemical research in toxicology 20050601 |
| Effects of vitamin E and pinoline on retinal lipid peroxidation. | Clinical & experimental optometry 20040501 |
| Binding of beta-carbolines at imidazoline I2 receptors: a structure-affinity investigation. | Bioorganic & medicinal chemistry letters 20040223 |
| Pyrazino[1,2-a]indoles as novel high-affinity and selective imidazoline I(2) receptor ligands. | Bioorganic & medicinal chemistry letters 20040223 |
| Binding of beta-carbolines at 5-HT(2) serotonin receptors. | Bioorganic & medicinal chemistry letters 20031215 |
| Effects of the beta-carbolines, harmane and pinoline, on insulin secretion from isolated human islets of Langerhans. | European journal of pharmacology 20031215 |
| Screening for endogenous substrates reveals that CYP2D6 is a 5-methoxyindolethylamine O-demethylase. | Pharmacogenetics 20030601 |
| Melatonin and pinoline prevent aluminium-induced lipid peroxidation in rat synaptosomes. | Journal of trace elements in medicine and biology : organ of the Society for Minerals and Trace Elements (GMS) 20030101 |
| Sustained ER Ca2+ depletion suppresses protein synthesis and induces activation-enhanced cell death in mast cells. | The Journal of biological chemistry 20020419 |