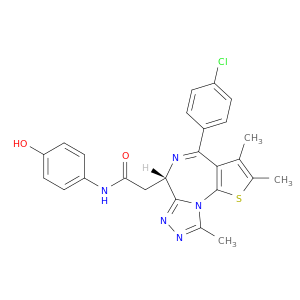
6H-Thieno[3,2-f][1,2,4]triazolo[4,3-a][1,4]diazepine-6-acetamide, 4-(4-chlorophenyl)-N-(4-hydroxyphenyl)-2,3,9-trimethyl-, (6S)-
| Title | Journal |
|---|---|
| The bromodomain inhibitor OTX015 (MK-8628) exerts anti-tumor activity in triple-negative breast cancer models as single agent and in combination with everolimus. | Oncotarget 20170131 |
| Promising in vivo efficacy of the BET bromodomain inhibitor OTX015/MK-8628 in malignant pleural mesothelioma xenografts. | International journal of cancer 20170101 |
| OTX015 (MK-8628), a novel BET inhibitor, exhibits antitumor activity in non-small cell and small cell lung cancer models harboring different oncogenic mutations. | Oncotarget 20161220 |
| Therapeutic efficacy of the bromodomain inhibitor OTX015/MK-8628 in ALK-positive anaplastic large cell lymphoma: an alternative modality to overcome resistant phenotypes. | Oncotarget 20161129 |
| OTX015 (MK-8628), a novel BET inhibitor, displays in vitro and in vivo antitumor effects alone and in combination with conventional therapies in glioblastoma models. | International journal of cancer 20161101 |
| Bromodomain inhibitor OTX015 (MK-8628) combined with targeted agents shows strong in vivo antitumor activity in lymphoma. | Oncotarget 20160906 |
| Bromodomain inhibitor OTX015 in patients with acute leukaemia: a dose-escalation, phase 1 study. | The Lancet. Haematology 20160401 |
| Phase I Population Pharmacokinetic Assessment of the Oral Bromodomain Inhibitor OTX015 in Patients with Haematologic Malignancies. | Clinical pharmacokinetics 20160301 |
| The BET inhibitor OTX015 reactivates latent HIV-1 through P-TEFb. | Scientific reports 20160101 |
| BET inhibitor OTX015 targets BRD2 and BRD4 and decreases c-MYC in acute leukemia cells. | Oncotarget 20150710 |
| Hijacking the E3 Ubiquitin Ligase Cereblon to Efficiently Target BRD4. | Chemistry & biology 20150618 |
| The BET Bromodomain Inhibitor OTX015 Affects Pathogenetic Pathways in Preclinical B-cell Tumor Models and Synergizes with Targeted Drugs. | Clinical cancer research : an official journal of the American Association for Cancer Research 20150401 |
| Compendium of aberrant DNA methylation and histone modifications in cancer. | Biochemical and biophysical research communications 20141205 |
| Targeting bromodomains: epigenetic readers of lysine acetylation. | Nature reviews. Drug discovery 20140501 |
| Targeting epigenetic readers in cancer. | The New England journal of medicine 20121101 |