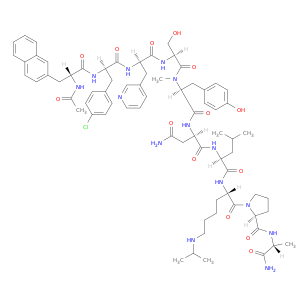
D-Alaninamide, N-acetyl-3-(2-naphthalenyl)-D-alanyl-4-chloro-D-phenylalanyl-3-(3-pyridinyl)-D-alanyl-L-seryl-N-methyl-L-tyrosyl-D-asparaginyl-L-leucyl-N6-(1-methylethyl)-L-lysyl-L-prolyl-
| Title | Journal |
|---|---|
| New treatment paradigm for prostate cancer: abarelix initiation therapy for immediate testosterone suppression followed by a luteinizing hormone-releasing hormone agonist. | BJU international 20120801 |
| Gonadotropin-releasing hormone: an update review of the antagonists versus agonists. | International journal of urology : official journal of the Japanese Urological Association 20120701 |
| Androgen deprivation therapy: past, present and future. | BJU international 20120601 |
| New treatment paradigm for prostate cancer: abarelix initiation therapy for immediate testosterone suppression followed by a luteinizing hormone-releasing hormone agonist. | BJU international 20120301 |
| Biochemistry, molecular biology and cell biology of gonadotropin-releasing hormone antagonists. | Current opinion in obstetrics & gynecology 20110801 |
| An update on the use of gonadotropin-releasing hormone antagonists in prostate cancer. | Therapeutic advances in urology 20110601 |
| Sipuleucel-T for therapy of asymptomatic or minimally symptomatic, castrate-refractory prostate cancer: an update and perspective among other treatments. | OncoTargets and therapy 20110101 |
| Androgen regulation of 5α-reductase isoenzymes in prostate cancer: implications for prostate cancer prevention. | PloS one 20110101 |
| Degarelix, a novel GnRH antagonist, causes minimal histamine release compared with cetrorelix, abarelix and ganirelix in an ex vivo model of human skin samples. | British journal of clinical pharmacology 20101001 |
| GnRH antagonists in the treatment of advanced prostate cancer. | The Canadian journal of urology 20100401 |
| Retraction statement: Reconstitution of Plenaxis® (Abarelix) 100 mg for injection is more effective with a vortex-like mixer than when performed manually. | Journal of pharmacy practice 20100201 |
| Evaluation of degarelix in the management of prostate cancer. | Cancer management and research 20100101 |
| Abarelix and other gonadotrophin-releasing hormone antagonists in prostate cancer. | BJU international 20091201 |
| [GnRH antagonists--a new therapy option for advanced prostate cancer]. | Aktuelle Urologie 20090501 |
| Will GnRH antagonists improve prostate cancer treatment? | Trends in endocrinology and metabolism: TEM 20090101 |
| Degarelix and its therapeutic potential in the treatment of prostate cancer. | Clinical interventions in aging 20090101 |
| Six-month depot formulation of leuprorelin acetate in the treatment of prostate cancer. | Clinical interventions in aging 20090101 |
| Leuprorelin depot injection: patient considerations in the management of prostatic cancer. | Therapeutics and clinical risk management 20080401 |
| Distinguishing compounds with anticancer activity by ANN using inductive QSAR descriptors. | Bioinformation 20080101 |
| Pharmacological characterization of a novel nonpeptide antagonist of the human gonadotropin-releasing hormone receptor, NBI-42902. | Endocrinology 20070201 |
| Hormone ablation therapy: lightening the load for today's prostate cancer patient. | Urologic nursing 20070201 |
| Abarelix for injectable suspension: first-in-class gonadotropin-releasing hormone antagonist for prostate cancer. | Future oncology (London, England) 20061201 |
| Dose-escalated abarelix in androgen-independent prostate cancer: a phase I study. | Anti-cancer drugs 20061001 |
| Proprietary Rel-Ease drug delivery technology: opportunity for sustained delivery of peptides, proteins and small molecules. | Expert opinion on drug delivery 20060901 |
| The effect of androgen deprivation therapy on fasting serum lipid and glucose parameters. | The Journal of urology 20060801 |
| Discovery of a pituitary adenoma following a gonadotropin-releasing hormone agonist in a patient with prostate cancer. | International journal of urology : official journal of the Japanese Urological Association 20060301 |
| Discovery of a pituitary adenoma following treatment with a gonadotropin-releasing hormone agonist in a patient with prostate cancer. | International journal of urology : official journal of the Japanese Urological Association 20060101 |
| [GnRH antagonists: present and future]. | Annales d'urologie 20051001 |
| Prostate-specific antigen decline after gonadotropin-releasing hormone antagonist withdrawal in androgen-independent prostate cancer. | Urology 20050401 |
| Innovations in antineoplastic therapy. | The Nursing clinics of North America 20050301 |
| Abarelix (plenaxis). | Clinical journal of oncology nursing 20041201 |
| Abarelix: the first gonadotrophin-releasing hormone antagonist for the treatment of prostate cancer. | Expert opinion on pharmacotherapy 20041001 |
| Hormone therapy in prostate cancer: LHRH antagonists versus LHRH analogues. | European urology 20040901 |
| Pharmacokinetics and pharmacodynamics of a novel depot formulation of abarelix, a gonadotropin-releasing hormone (GnRH) antagonist, in healthy men ages 50 to 75. | Journal of clinical pharmacology 20040501 |
| Abarelix (Plenaxis) for advanced prostate cancer. | The Medical letter on drugs and therapeutics 20040315 |
| Abarelix (Plenaxis): a gonadotropin-releasing hormone antagonist for medical castration in patients with advanced prostate cancer. | Clinical prostate cancer 20040301 |
| Targeting FSH in androgen-independent prostate cancer: abarelix for prostate cancer progressing after orchiectomy. | Urology 20040201 |
| Plenaxis. | Discovery medicine 20040201 |
| New treatment for advanced prostate cancer. | FDA consumer 20040101 |
| Gonadotropin-releasing hormone antagonist in the management of prostate cancer. | Reviews in urology 20040101 |
| Experimental use of GnRH antagonists as second-line hormonal therapy. | Reviews in urology 20040101 |
| Luteinizing hormone-releasing hormone agonists in the treatment of men with prostate cancer: timing, alternatives, and the 1-year implant. | Urology 20031222 |
| An open-label study of abarelix in men with symptomatic prostate cancer at risk of treatment with LHRH agonists. | Urology 20031101 |
| Gateways to clinical trials. | Methods and findings in experimental and clinical pharmacology 20031101 |
| Phase II study of abarelix depot for androgen independent prostate cancer progression during gonadotropin-releasing hormone agonist therapy. | The Journal of urology 20030501 |
| Pharmacokinetics and pharmacodynamics of abarelix, a gonadotropin-releasing hormone antagonist, after subcutaneous continuous infusion in patients with prostate cancer. | Clinical pharmacology and therapeutics 20030401 |
| Encouraging results for Plenaxis in prostate cancer trial. | Expert review of anticancer therapy 20030401 |
| Abarelix: abarelix-depot-F, abarelix-depot-M, abarelix-L, PPI 149, R 3827. | Drugs in R&D 20030101 |
| Gateways to clinical trials. | Methods and findings in experimental and clinical pharmacology 20021101 |
| Hormonal therapy of prostate cancer. | Seminars in urologic oncology 20020801 |
| Gateways to Clinical Trials. June 2002. | Methods and findings in experimental and clinical pharmacology 20020601 |
| Gateways to clinical trials. | Methods and findings in experimental and clinical pharmacology 20020501 |
| Pharmacological profile of a new, potent, and long-acting gonadotropin-releasing hormone antagonist: degarelix. | The Journal of pharmacology and experimental therapeutics 20020401 |
| A phase 3, multicenter, open label, randomized study of abarelix versus leuprolide plus daily antiandrogen in men with prostate cancer. | The Journal of urology 20020401 |
| New single-isomer compounds on the horizon. | CNS spectrums 20020401 |
| A phase 3, multicenter, open-label, randomized study of abarelix versus leuprolide acetate in men with prostate cancer. | Urology 20011101 |
| Luteinizing hormone-releasing hormone antagonists in prostate cancer. | Urology 20010801 |
| The gonadotropin-releasing hormone antagonist abarelix depot versus luteinizing hormone releasing hormone agonists leuprolide or goserelin: initial results of endocrinological and biochemical efficacies in patients with prostate cancer. | The Journal of urology 20010501 |
| GnRH antagonists: a new generation of long acting analogues incorporating p-ureido-phenylalanines at positions 5 and 6. | Journal of medicinal chemistry 20010201 |
| Androgen deprivation and other treatments for advanced prostate cancer. | Reviews in urology 20010101 |
| The evolution of hormonal therapy for prostatic carcinoma. | Reviews in urology 20010101 |
| Emerging pharmacologic therapies for prostate cancer. | Reviews in urology 20010101 |