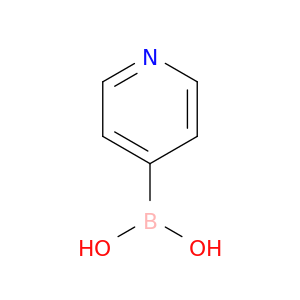
Pyridine-4-boronic acid
| Title | Journal |
|---|---|
| Synthesis, X-ray analysis, and biological evaluation of a new class of stereopure lactam-based HIV-1 protease inhibitors. | Journal of medicinal chemistry 20120322 |
| Discovery of 1,2,4-triazine derivatives as adenosine A(2A) antagonists using structure based drug design. | Journal of medicinal chemistry 20120308 |
| Toward new therapeutics for skin and soft tissue infections: propargyl-linked antifolates are potent inhibitors of MRSA and Streptococcus pyogenes. | PloS one 20120101 |
| Synthesis of functionalized cinnamaldehyde derivatives by an oxidative Heck reaction and their use as starting materials for preparation of Mycobacterium tuberculosis 1-deoxy-D-xylulose-5-phosphate reductoisomerase inhibitors. | The Journal of organic chemistry 20111104 |
| trans-Dichloridobis[(pyridin-4-yl)boronic acid-κN]palladium(II) dimethyl sulfoxide disolvate. | Acta crystallographica. Section E, Structure reports online 20110501 |
| Synthesis, carbohydrate- and DNA-binding studies of cationic 2,2':6',2''-terpyridineplatinum(II) complexes containing N- and S-donor boronic acid ligands. | Dalton transactions (Cambridge, England : 2003) 20110114 |
| Novel tricyclic pyrazole BRAF inhibitors with imidazole or furan central scaffolds. | Bioorganic & medicinal chemistry 20100915 |
| Metal-free carbon-carbon bond-forming reductive coupling between boronic acids and tosylhydrazones. | Nature chemistry 20090901 |
| An experimental and theoretical study of molecular structure and vibrational spectra of 3- and 4-pyridineboronic acid molecules by density functional theory calculations. | Spectrochimica acta. Part A, Molecular and biomolecular spectroscopy 20080801 |
| Synthesis and preliminary DNA-binding studies of diimineplatinum(II) complexes containing 3- or 4-pyridineboronic acid. | Dalton transactions (Cambridge, England : 2003) 20070607 |
| A novel p38 alpha MAPK inhibitor suppresses brain proinflammatory cytokine up-regulation and attenuates synaptic dysfunction and behavioral deficits in an Alzheimer's disease mouse model. | Journal of neuroinflammation 20070101 |
| N-alkyl-4-boronopyridinium salts as thermally stable and reusable amide condensation catalysts. | Organic letters 20051027 |
| N-alkyl-4-boronopyridinium halides versus boric acid as catalysts for the esterification of alpha-hydroxycarboxylic acids. | Organic letters 20051027 |
| A molecular box derived from cobaloxime units held together by 4-pyridinylboronic acid residues. | Inorganic chemistry 20011022 |