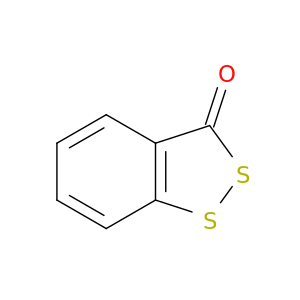
3H-Benzo[c][1,2]dithiol-3-one
| Title | Journal |
|---|---|
| Thiol-dependent DNA cleavage by aminomethylated Beaucage's reagent. | Organic & biomolecular chemistry 20100321 |
| Solid-supported reagents for synthesis of nucleoside monothiophosphates, dithiodiphosphates, and trithiotriphosphates. | Current protocols in nucleic acid chemistry 20090301 |
| Possible chemical mechanisms underlying the antitumor activity of S-deoxyleinamycin. | Bioorganic & medicinal chemistry letters 20080515 |
| Substituent effects on the reactivity of benzo-1,2-dithiolan-3-one 1-oxides and their possible application to the synthesis of DNA-targeting drugs. | The Journal of organic chemistry 20050819 |