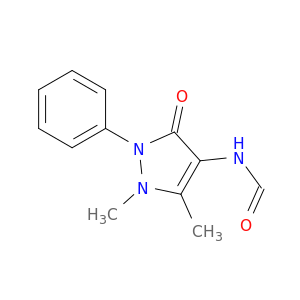
(1,5-Dimethyl-3-oxo-2-phenyl-2,3-dihydro-1h-pyrazol-4-yl)formamide
| Title | Journal |
|---|---|
| High-performance liquid chromatographic assay for metamizol metabolites in rat plasma: application to pharmacokinetic studies. | Journal of pharmaceutical and biomedical analysis 20121201 |
| N-(1,5-Dimethyl-3-oxo-2-phenyl-2,3-dihydro-1H-pyrazol-4-yl)formamide. | Acta crystallographica. Section E, Structure reports online 20110601 |
| Synthesis and SAR studies of 1,4-benzoxazine MenB inhibitors: novel antibacterial agents against Mycobacterium tuberculosis. | Bioorganic & medicinal chemistry letters 20101101 |
| Investigation of the microbial degradation of phenazone-type drugs and their metabolites by natural biofilms derived from river water using liquid chromatography/tandem mass spectrometry (LC-MS/MS). | Water research 20100801 |
| Hydrophilic anthropogenic markers for quantification of wastewater contamination in ground- and surface waters. | Environmental toxicology and chemistry 20091201 |
| Photodegradation study of three dipyrone metabolites in various water systems: identification and toxicity of their photodegradation products. | Water research 20080501 |
| Occurrence, fate and assessment of polar metamizole (dipyrone) residues in hospital and municipal wastewater. | Chemosphere 20080401 |
| Behaviour and redox sensitivity of pharmaceutical residues during bank filtration - Investigation of residues of phenazone-type analgesics. | Chemosphere 20080401 |
| Clinical review: Drug metabolism and nonrenal clearance in acute kidney injury. | Critical care (London, England) 20080101 |
| Investigation of the behavior and metabolism of pharmaceutical residues during purification of contaminated ground water used for drinking water supply. | Chemosphere 20071101 |
| [Analysis of three metabolite residues of dipyrone in bovine muscle and pork muscle using high performance liquid chromatography]. | Se pu = Chinese journal of chromatography 20071101 |
| Long-term comparison of trace organics removal performances between conventional and membrane activated sludge processes. | Water environment research : a research publication of the Water Environment Federation 20061201 |
| Simultaneous determination of residues of dipyrone and its major metabolites in milk, bovine muscle, and porcine muscle by liquid chromatography/mass spectrometry. | Journal of AOAC International 20050101 |
| Pharmaceuticals in the river Elbe and its tributaries. | Chemosphere 20041001 |
| Allergic cholestatic hepatitis and exanthema induced by metamizole: verification by lymphocyte transformation test. | Liver 20021201 |
| Impairment of the metabolism of dipyrone in asymptomatic carriers of the hepatitis-B virus does not occur in rapid acetylators. | European journal of clinical pharmacology 20010901 |