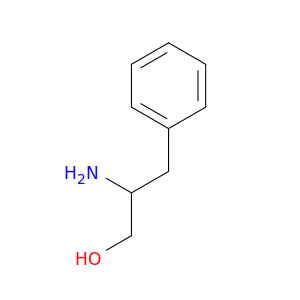
Benzenepropanol, β-amino-
| Title | Journal |
|---|---|
| A colorimetric chiral sensor based on chiral crown ether for the recognition of the two enantiomers of primary amino alcohols and amines. | Chirality 20110401 |
| Synthesis and SAR studies of 1,4-benzoxazine MenB inhibitors: novel antibacterial agents against Mycobacterium tuberculosis. | Bioorganic & medicinal chemistry letters 20101101 |
| Direct high-performance liquid chromatographic separation of the enantiomers of an aromatic amine and four aminoalcohols using polysaccharide chiral stationary phases and acidic additive. | Chirality 20070801 |
| Straightforward methodology for the enantioselective synthesis of benzo[a]- and indolo[2,3-a]quinolizidines. | The Journal of organic chemistry 20070706 |
| Synthesis and activity of 2-oxoamides containing long chain beta-amino acids. | Journal of peptide science : an official publication of the European Peptide Society 20050701 |
| Cytotoxicity of enantiomers of gossypol Schiff's bases and optical stability of gossypolone. | European journal of medicinal chemistry 20040701 |
| Chiral bis(amino alcohol)oxalamide gelators-gelation properties and supramolecular organization: racemate versus pure enantiomer gelation. | Chemistry (Weinheim an der Bergstrasse, Germany) 20031121 |
| Peptide inhibitors of CDK2-cyclin A that target the cyclin recruitment-site: structural variants of the C-terminal Phe. | Bioorganic & medicinal chemistry letters 20020916 |
| Optically active BINOL core-based phenyleneethynylene dendrimers for the enantioselective fluorescent recognition of amino alcohols. | The Journal of organic chemistry 20010907 |
| Chemotactic peptide analogues. Synthesis and chemotactic activity of N-formyl-Met-Leu-Phe analogues containing (S)-phenylalaninol derivatives. | Archiv der Pharmazie 19950901 |