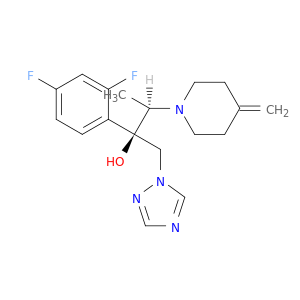
(2R,3R)-2-(2,4-Difluorophenyl)-3-(4-methylenepiperidin-1-yl)-1-(1h-1,2,4-triazol-1-yl)butan-2-ol
| Title | Journal |
|---|---|
| Efinaconazole: first global approval. | Drugs 20131101 |
| Mechanism of action of efinaconazole, a novel triazole antifungal agent. | Antimicrobial agents and chemotherapy 20130501 |
| KP-103, a novel triazole derivative, is effective in preventing relapse and successfully treating experimental interdigital tinea pedis and tinea corporis in guinea pigs. | Microbiology and immunology 20020101 |