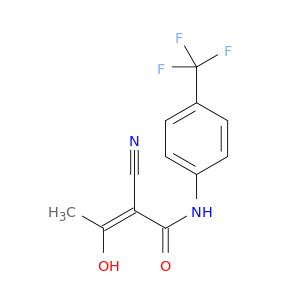
Teriflunomide
| Title | Journal |
|---|---|
| Mitochondrial dysfunction induced by leflunomide and its active metabolite. | Toxicology 20180301 |
| Fingerprinting of neurotoxic compounds using a mouse embryonic stem cell dual luminescence reporter assay. | Archives of toxicology 20170101 |
| Long-term safety and efficacy of teriflunomide: Nine-year follow-up of the randomized TEMSO study. | Neurology 20160308 |
| Systems pharmacological analysis of drugs inducing stevens-johnson syndrome and toxic epidermal necrolysis. | Chemical research in toxicology 20150518 |
| Novel selective and potent inhibitors of malaria parasite dihydroorotate dehydrogenase: discovery and optimization of dihydrothiophenone derivatives. | Journal of medicinal chemistry 20131024 |
| On dihydroorotate dehydrogenases and their inhibitors and uses. | Journal of medicinal chemistry 20130425 |
| Discovery of diverse human dihydroorotate dehydrogenase inhibitors as immunosuppressive agents by structure-based virtual screening. | Journal of medicinal chemistry 20121011 |
| New inhibitors of dihydroorotate dehydrogenase (DHODH) based on the 4-hydroxy-1,2,5-oxadiazol-3-yl (hydroxyfurazanyl) scaffold. | European journal of medicinal chemistry 20120301 |
| Biaryl analogues of teriflunomide as potent DHODH inhibitors. | Bioorganic & medicinal chemistry letters 20111215 |
| Hepatic cytochrome P450s attenuate the cytotoxicity induced by leflunomide and its active metabolite A77 1726 in primary cultured rat hepatocytes. | Toxicological sciences : an official journal of the Society of Toxicology 20110801 |
| 1,2,5-Oxadiazole analogues of leflunomide and related compounds. | European journal of medicinal chemistry 20110101 |
| Structure-based design, synthesis, and characterization of inhibitors of human and Plasmodium falciparum dihydroorotate dehydrogenases. | Journal of medicinal chemistry 20090514 |
| ATP competitive inhibitors of D-alanine-D-alanine ligase based on protein kinase inhibitor scaffolds. | Bioorganic & medicinal chemistry 20090201 |
| Dihydroorotate dehydrogenase inhibitor A771726 (leflunomide) induces apoptosis and diminishes proliferation of multiple myeloma cells. | Molecular cancer therapeutics 20090201 |
| Spotlight on teriflunomide. | International MS journal 20080601 |
| The immunomodulatory drug Leflunomide inhibits cell cycle progression of B-CLL cells. | Leukemia 20080301 |
| Design and synthesis of potent inhibitors of the malaria parasite dihydroorotate dehydrogenase. | Journal of medicinal chemistry 20070125 |
| A Phase II study of the safety and efficacy of teriflunomide in multiple sclerosis with relapses. | Neurology 20060328 |
| SAR, species specificity, and cellular activity of cyclopentene dicarboxylic acid amides as DHODH inhibitors. | Bioorganic & medicinal chemistry letters 20051101 |
| [The active metabolite of leflunomide A771726 inhibits proliferation and collagen synthesis of hepatic stellate cell]. | Zhejiang da xue xue bao. Yi xue ban = Journal of Zhejiang University. Medical sciences 20041101 |
| A77 1726 induces differentiation of human myeloid leukemia K562 cells by depletion of intracellular CTP pools. | Molecular pharmacology 20020901 |
| Inhibition of HIV replication by A77 1726, the active metabolite of leflunomide, in combination with pyrimidine nucleoside reverse transcriptase inhibitors. | Journal of acquired immune deficiency syndromes (1999) 20011001 |
| Recombinant expression of N-terminal truncated mutants of the membrane bound mouse, rat and human flavoenzyme dihydroorotate dehydrogenase. A versatile tool to rate inhibitor effects? | European journal of biochemistry 20010301 |
| New dermatological agents for the treatment of psoriasis. | Journal of medicinal chemistry 20010201 |
| Immunosuppressive leflunomide metabolite (A77 1726) blocks TNF-dependent nuclear factor-kappa B activation and gene expression. | Journal of immunology (Baltimore, Md. : 1950) 19990215 |
| Species-related inhibition of human and rat dihydroorotate dehydrogenase by immunosuppressive isoxazol and cinchoninic acid derivatives. | Biochemical pharmacology 19981101 |
| Structural and functional comparison of agents interfering with dihydroorotate, succinate and NADH oxidation of rat liver mitochondria. | Biochemical pharmacology 19981015 |
| Differential modulation of pro- and anti-inflammatory cytokine receptors by N-(4-trifluoromethylphenyl)-2-cyano-3-hydroxy-crotonic acid amide (A77 1726), the physiologically active metabolite of the novel immunomodulator leflunomide. | Biochemical pharmacology 19980501 |