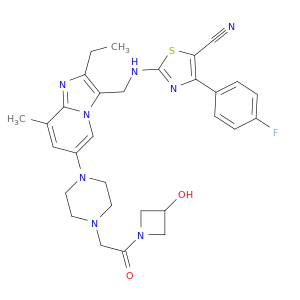
GLPG1690
| Title | Journal |
|---|---|
| Discovery of 2-[[2-Ethyl-6-[4-[2-(3-hydroxyazetidin-1-yl)-2-oxoethyl]piperazin-1-yl]-8-methylimidazo[1,2-a]pyridin-3-yl]methylamino]-4-(4-fluorophenyl)thiazole-5-carbonitrile (GLPG1690), a First-in-Class Autotaxin Inhibitor Undergoing Clinical Evaluation for the Treatment of Idiopathic Pulmonary Fibrosis. | Journal of medicinal chemistry 20170511 |
| Selective Inhibition of Autotaxin Is Efficacious in Mouse Models of Liver Fibrosis. | The Journal of pharmacology and experimental therapeutics 20170101 |
| The autotaxin-lysophosphatidic acid pathway in pathogenesis of rheumatoid arthritis. | European journal of pharmacology 20151015 |
| Autotaxin production of lysophosphatidic acid mediates allergic asthmatic inflammation. | American journal of respiratory and critical care medicine 20131015 |
| Lysophosphatidic acid (LPA) and its receptors: role in airway inflammation and remodeling. | Biochimica et biophysica acta 20130101 |
| Problems of the pathogenesis, clinics, and therapy of panarteritis of the aorta and its branches. | Cor et vasa 19750101 |