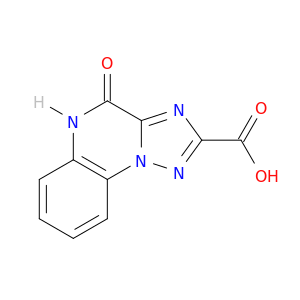
[1,2,4]Triazolo[1,5-a]quinoxaline-2-carboxylic acid, 4,5-dihydro-4-oxo-
| Title | Journal |
|---|---|
| Synthesis and biological evaluation of novel 9-heteroaryl substituted 7-chloro-4,5-dihydro-4-oxo-1,2,4-triazolo[1,5-a]quinoxaline-2-carboxylates (TQX) as (R,S)-2-amino-3-(3-hydroxy-5-methylisoxazol-4-yl)propionic acid (AMPA) receptor antagonists. | Chemical & pharmaceutical bulletin 20080801 |
| 1,2,4-Triazolo[1,5-a]quinoxaline as a versatile tool for the design of selective human A3 adenosine receptor antagonists: synthesis, biological evaluation, and molecular modeling studies of 2-(hetero)aryl- and 2-carboxy-substituted derivatives. | Journal of medicinal chemistry 20051215 |
| Selectivity fields: comparative molecular field analysis (CoMFA) of the glycine/NMDA and AMPA receptors. | Journal of medicinal chemistry 20030911 |