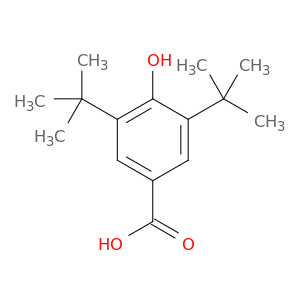
3,5-Di-tert-butyl-4-hydroxybenzoic acid
| Title | Journal |
|---|---|
| New losartan-hydrocaffeic acid hybrids as antihypertensive-antioxidant dual drugs: Ester, amide and amine linkers. | European journal of medicinal chemistry 20120401 |
| Synthesis and SAR studies of 1,4-benzoxazine MenB inhibitors: novel antibacterial agents against Mycobacterium tuberculosis. | Bioorganic & medicinal chemistry letters 20101101 |
| Determination of synthetic phenolic antioxidants and their metabolites in water samples by downscaled solid-phase extraction, silylation and gas chromatography-mass spectrometry. | Journal of chromatography. A 20101008 |
| Novel compounds designed as antistress agents. | Journal of medicinal chemistry 20091126 |
| Toxicity and effects of 2,6-di-tert-butyl-4-methylphenyl N-methylcarbamate (terbutol) on hepatic cytochrome P450 in F344 rats. | Archives of toxicology 20011101 |
| Synergistic protective effects of antioxidant and nitric oxide synthase inhibitor in transient focal ischemia. | Journal of cerebral blood flow and metabolism : official journal of the International Society of Cerebral Blood Flow and Metabolism 19990201 |