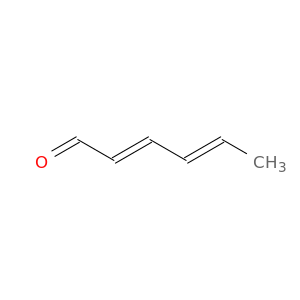
2,4-Hexadienal
| Title | Journal |
|---|---|
| SENS-IS, a 3D reconstituted epidermis based model for quantifying chemical sensitization potency: Reproducibility and predictivity results from an inter-laboratory study. | Toxicology in vitro : an international journal published in association with BIBRA 20160401 |
| Population-based in vitro hazard and concentration-response assessment of chemicals: the 1000 genomes high-throughput screening study. | Environmental health perspectives 20150501 |
| Chemicals inducing acute irritant contact dermatitis mobilize intracellular calcium in human keratinocytes. | Toxicology in vitro : an international journal published in association with BIBRA 20130201 |
| exo-Selective asymmetric Diels-Alder reaction of 2,4-dienals and nitroalkenes by trienamine catalysis. | Angewandte Chemie (International ed. in English) 20110905 |
| Human aldo-keto reductases 1B1 and 1B10: a comparative study on their enzyme activity toward electrophilic carbonyl compounds. | Chemico-biological interactions 20110530 |
| Characterization of Taenia solium cysticerci microsomal glutathione S-transferase activity. | Parasitology research 20071001 |
| The atmospheric photolysis of E-2-hexenal, Z-3-hexenal and E,E-2,4-hexadienal. | Physical chemistry chemical physics : PCCP 20061128 |
| Volatile constituents from the leaves of Callicarpa japonica Thunb. and their antibacterial activities. | Journal of agricultural and food chemistry 20040225 |
| NTP toxicology and carcinogensis Studies of 2,4-hexadienal (89% trans,trans isomer, CAS No. 142-83-6; 11% cis,trans isomer) (Gavage Studies). | National Toxicology Program technical report series 20031001 |
| Forestomach tumor induction by 2,4-hexadienal in F344N rats and B6C3F1 mice. | Archives of toxicology 20030901 |
| Alpha,beta-unsaturated carbonyl compounds: induction of oxidative DNA damage in mammalian cells. | Mutagenesis 20030901 |
| Short term feeding of hexadienal to postnatal rats: effects on stomach aldehyde dehydrogenase. | Toxicology letters 20030113 |
| Glutathione S-transferase pi expression in forestomach carcinogenesis process induced by gavage-administered 2,4-hexadienal in the F344 rat. | Archives of toxicology 20011201 |
| Structural and kinetic determinants of aldehyde reduction by aldose reductase. | Biochemistry 19990105 |