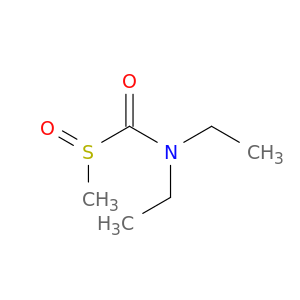
Formamide, N,N-diethyl-1-(methylsulfinyl)-
| Title | Journal |
|---|---|
| Antitubercular activity of disulfiram, an antialcoholism drug, against multidrug- and extensively drug-resistant Mycobacterium tuberculosis isolates. | Antimicrobial agents and chemotherapy 20120801 |
| Persistent pain is dependent on spinal mitochondrial antioxidant levels. | The Journal of neuroscience : the official journal of the Society for Neuroscience 20090107 |
| Disulfiram metabolites permanently inactivate the human multidrug resistance P-glycoprotein. | Molecular pharmaceutics 20040101 |
| Chemical modifications to study mutations that affect the ability of the general base (E268) to function in human liver mitochondrial aldehyde dehydrogenase. | Chemico-biological interactions 20030201 |
| Metabolism of a disulfiram metabolite, S-methyl N,N-diethyldithiocarbamate, by flavin monooxygenase in human renal microsomes. | Drug metabolism and disposition: the biological fate of chemicals 20010201 |
| Overview--in vitro inhibition of aldehyde dehydrogenase by disulfiram and metabolites. | Chemico-biological interactions 20010130 |
| In vivo inhibition of aldehyde dehydrogenase by disulfiram. | Chemico-biological interactions 20010130 |
| S-methyl-N,N-diethylthiocarbamate sulfoxide elicits neuroprotective effect against N-methyl-D-aspartate receptor-mediated neurotoxicity. | Journal of biomedical science 20010101 |
| Rat liver constitutive and phenobarbital-inducible cytosolic aldehyde dehydrogenases are highly homologous proteins that function as distinct isozymes. | Biochemistry 20000912 |