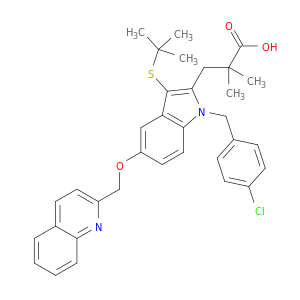
1H-Indole-2-propanoic acid, 1-[(4-chlorophenyl)methyl]-3-[(1,1-dimethylethyl)thio]-α,α-dimethyl-5-(2-quinolinylmethoxy)-
| Title | Journal |
|---|---|
| Lead modification: amino acid appended indoles as highly effective 5-LOX inhibitors. | Bioorganic & medicinal chemistry 20140301 |
| MK591, a leukotriene biosynthesis inhibitor, induces apoptosis in prostate cancer cells: synergistic action with LY294002, an inhibitor of phosphatidylinositol 3'-kinase. | Cancer letters 20100528 |
| 5-Lipoxygenase-activating protein inhibitors. Part 2: 3-{5-((S)-1-Acetyl-2,3-dihydro-1H-indol-2-ylmethoxy)-3-tert-butylsulfanyl-1-[4-(5-methoxy-pyrimidin-2-yl)-benzyl]-1H-indol-2-yl}-2,2-dimethyl-propionic acid (AM679)--a potent FLAP inhibitor. | Bioorganic & medicinal chemistry letters 20100101 |
| 5-lipoxygenase-activating protein inhibitors: development of 3-[3-tert-butylsulfanyl-1-[4-(6-methoxy-pyridin-3-yl)-benzyl]-5-(pyridin-2-ylmethoxy)-1H-indol-2-yl]-2,2-dimethyl-propionic acid (AM103). | Journal of medicinal chemistry 20091008 |
| Effect of 5-lipoxygenase inhibitor MK591 on early molecular and signaling events induced by staphylococcal enterotoxin B in human peripheral blood mononuclear cells. | The FEBS journal 20080601 |
| Substituted 2,2-bisaryl-bicycloheptanes as novel and potent inhibitors of 5-lipoxygenase activating protein. | Bioorganic & medicinal chemistry letters 20080315 |
| Angiotensin II-induced abdominal aortic aneurysm occurs independently of the 5-lipoxygenase pathway in apolipoprotein E-deficient mice. | Prostaglandins & other lipid mediators 20070801 |
| Crystal structure of inhibitor-bound human 5-lipoxygenase-activating protein. | Science (New York, N.Y.) 20070727 |
| Montelukast regulates eosinophil protease activity through a leukotriene-independent mechanism. | The Journal of allergy and clinical immunology 20060701 |
| Arachidonic acid activates phospholipase D in human neutrophils; essential role of endogenous leukotriene B4 and inhibition by adenosine A2A receptor engagement. | Journal of leukocyte biology 20030401 |
| Role of 5-lipoxygenase products in the local accumulation of neutrophils in dermal inflammation in the rabbit. | Journal of immunology (Baltimore, Md. : 1950) 19990915 |
| Assessment of the in vivo biochemical efficacy of orally active leukotriene biosynthesis inhibitors. | Agents and actions 19930901 |