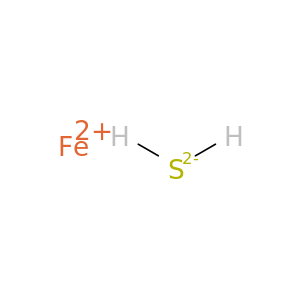
Iron sulfide (FeS)
| Title | Journal |
|---|---|
| Enhanced reductive dechlorination of tetrachloroethene during reduction of cobalamin (III) by nano-mackinawite. | Journal of hazardous materials 20121015 |
| Carbon isotope fractionation in reactions of 1,2-dibromoethane with FeS and hydrogen sulfide. | Environmental science & technology 20120717 |
| Controlled soft-template synthesis of ultrathin C@FeS nanosheets with high-Li-storage performance. | ACS nano 20120626 |
| Synthesis of FeS and FeSe nanoparticles from a single source precursor: a study of their photocatalytic activity, peroxidase-like behavior, and electrochemical sensing of H2O2. | ACS applied materials & interfaces 20120401 |
| Uranium(VI) reduction by iron(II) monosulfide mackinawite. | Environmental science & technology 20120320 |
| Removal of highly elevated nitrate from drinking water by pH-heterogenized heterotrophic denitrification facilitated with ferrous sulfide-based autotrophic denitrification. | Bioresource technology 20111101 |
| FeS/S/FeS(2) redox system and its oxidoreductase-like chemistry in the iron-sulfur world. | Astrobiology 20110601 |
| Facile Synthesis and Characterization of Fe/FeS Nanoparticles for Environmental Applications. | ACS applied materials & interfaces 20110501 |
| Probing the role of the bridging C509 between the [Fe4S4] cubane and the [Ni(p)Ni(d)] centre in the A-cluster of acetyl-coenzyme A synthase. | Chemical communications (Cambridge, England) 20110128 |
| Biological reduction of uranium--from the laboratory to the field. | The Science of the total environment 20101115 |
| Comment on 'Reductive immobilization of uranium(VI) by amorphous iron sulfide'. | Environmental science & technology 20090215 |
| Nanostructured FeS as a mimic peroxidase for biocatalysis and biosensing. | Chemistry (Weinheim an der Bergstrasse, Germany) 20090101 |
| Stabilization of fully reduced iron-sulfur clusters by carbene ligation: the [FenSn]0 oxidation levels (n = 4, 8). | Journal of the American Chemical Society 20080730 |
| Nucleic acids bind to nanoparticulate iron (II) monosulphide in aqueous solutions. | Origins of life and evolution of the biosphere : the journal of the International Society for the Study of the Origin of Life 20080601 |
| Reductive dechlorination pathways of tetrachloroethylene and trichloroethylene and subsequent transformation of their dechlorination products by mackinawite (FeS) in the presence of metals. | Environmental science & technology 20071115 |
| Reductive dechlorination of tetrachloroethylene and trichloroethylene by mackinawite (FeS) in the presence of metals: reaction rates. | Environmental science & technology 20070915 |
| Abiotic transformation of DDT in aqueous solutions. | Chemosphere 20061101 |
| Computational studies of the H-cluster of Fe-only hydrogenases: geometric, electronic, and magnetic properties and their dependence on the [Fe4S4] cubane. | Inorganic chemistry 20051212 |
| Carbon isotope fractionation in the reductive dehalogenation of carbon tetrachloride at iron (hydr)oxide and iron sulfide minerals. | Environmental science & technology 20050801 |
| Ammonia formation by the reduction of nitrite/nitrate by FeS: ammonia formation under acidic conditions. | Origins of life and evolution of the biosphere : the journal of the International Society for the Study of the Origin of Life 20050801 |
| Similarities between inorganic sulfide and the strong Hg(II)-complexing ligands in municipal wastewater effluent. | Environmental science & technology 20050601 |
| Impact of transition metals on reductive dechlorination rate of hexachloroethane by mackinawite. | Environmental science & technology 20031015 |
| A possible primordial peptide cycle. | Science (New York, N.Y.) 20030815 |
| Effects of FeS on the transformation kinetics of gamma-hexachlorcyclohexane. | Environmental science & technology 20030501 |
| Kinetics of 1,1,1-trichloroethane transformation by iron sulfide and a methanogenic consortium. | Environmental science & technology 20021101 |
| Factors influencing rates and products in the transformation of trichloroethylene by iron sulfide and iron metal. | Environmental science & technology 20011001 |