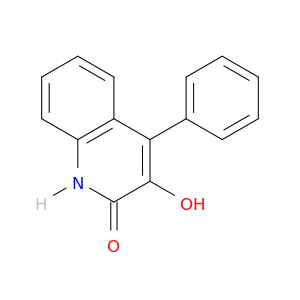
2(1H)-Quinolinone, 3-hydroxy-4-phenyl-
| Title | Journal |
|---|---|
| Non-heme dioxygenase catalyzes atypical oxidations of 6,7-bicyclic systems to form the 6,6-quinolone core of viridicatin-type fungal alkaloids. | Angewandte Chemie (International ed. in English) 20141117 |
| Phylogeny of Penicillium and the segregation of Trichocomaceae into three families. | Studies in mycology 20111115 |
| Phylogeny and nomenclature of the genus Talaromyces and taxa accommodated in Penicillium subgenus Biverticillium. | Studies in mycology 20111115 |
| 3-Hy-droxy-4-(3-hy-droxy-phen-yl)-2-quinolone monohydrate. | Acta crystallographica. Section E, Structure reports online 20110801 |
| [Efficient construction of nitrogen-containing heterocycles utilizing CN functional groups and its application to the synthesis of natural products]. | Yakugaku zasshi : Journal of the Pharmaceutical Society of Japan 20110101 |
| A concise and versatile synthesis of viridicatin alkaloids from cyanoacetanilides. | Organic letters 20090402 |
| [The biosynthesis of low-molecular-weight nitrogen-containing secondary metabolite-alkaloids by the resident strains of Penicillium chrysogenum and Penicillium expansum isolated on the board of the Mir space station ]. | Mikrobiologiia 20020101 |
| Isolation and characterization of the fungal metabolite 3-O-methylviridicatin as an inhibitor of tumour necrosis factor alpha-induced human immunodeficiency virus replication. | Antiviral chemistry & chemotherapy 19980301 |