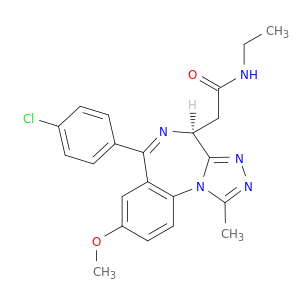
4H-[1,2,4]Triazolo[4,3-a][1,4]benzodiazepine-4-acetamide, 6-(4-chlorophenyl)-N-ethyl-8-methoxy-1-methyl-, (4S)-
| Title | Journal |
|---|---|
| Inhibition of histone H3K27 demethylases selectively modulates inflammatory phenotypes of natural killer cells. | The Journal of biological chemistry 20180216 |
| The BET inhibitor JQ1 selectively impairs tumour response to hypoxia and downregulates CA9 and angiogenesis in triple negative breast cancer. | Oncogene 20170105 |
| MCM5 as a target of BET inhibitors in thyroid cancer cells. | Endocrine-related cancer 20160401 |
| CCAT1 is an enhancer-templated RNA that predicts BET sensitivity in colorectal cancer. | The Journal of clinical investigation 20160201 |
| BET inhibitor resistance emerges from leukaemia stem cells. | Nature 20150924 |
| An epigenomic approach to therapy for tamoxifen-resistant breast cancer. | Cell research 20140701 |
| Targeting STAT5 in hematologic malignancies through inhibition of the bromodomain and extra-terminal (BET) bromodomain protein BRD2. | Molecular cancer therapeutics 20140501 |
| Potent antimyeloma activity of the novel bromodomain inhibitors I-BET151 and I-BET762. | Blood 20140130 |
| Discovery, Design, and Optimization of Isoxazole Azepine BET Inhibitors. | ACS medicinal chemistry letters 20130912 |
| Optimization of 3,5-dimethylisoxazole derivatives as potent bromodomain ligands. | Journal of medicinal chemistry 20130425 |
| Cancer research: Open ambition. | Nature 20120809 |
| From ApoA1 upregulation to BET family bromodomain inhibition: discovery of I-BET151. | Bioorganic & medicinal chemistry letters 20120415 |
| Fragment-based discovery of bromodomain inhibitors part 2: optimization of phenylisoxazole sulfonamides. | Journal of medicinal chemistry 20120126 |
| BET bromodomain inhibition as a therapeutic strategy to target c-Myc. | Cell 20110916 |
| Liver X receptors as regulators of macrophage inflammatory and metabolic pathways. | Biochimica et biophysica acta 20110801 |
| Discovery and characterization of small molecule inhibitors of the BET family bromodomains. | Journal of medicinal chemistry 20110609 |
| Suppression of inflammation by a synthetic histone mimic. | Nature 20101223 |