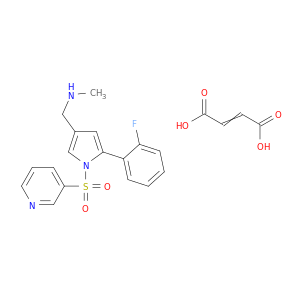
TAK-438
| Title | Journal |
|---|---|
| Discovery of a novel pyrrole derivative 1-[5-(2-fluorophenyl)-1-(pyridin-3-ylsulfonyl)-1H-pyrrol-3-yl]-N-methylmethanamine fumarate (TAK-438) as a potassium-competitive acid blocker (P-CAB). | Journal of medicinal chemistry 20120510 |
| High-throughput screening of potassium-competitive acid blockers. | Journal of biomolecular screening 20120201 |
| Characterization of a novel potassium-competitive acid blocker of the gastric H,K-ATPase, 1-[5-(2-fluorophenyl)-1-(pyridin-3-ylsulfonyl)-1H-pyrrol-3-yl]-N-methylmethanamine monofumarate (TAK-438). | The Journal of pharmacology and experimental therapeutics 20111101 |
| A study comparing the antisecretory effect of TAK-438, a novel potassium-competitive acid blocker, with lansoprazole in animals. | The Journal of pharmacology and experimental therapeutics 20110601 |
| A comparative study on the modes of action of TAK-438, a novel potassium-competitive acid blocker, and lansoprazole in primary cultured rabbit gastric glands. | Biochemical pharmacology 20110501 |
| 1-[5-(2-Fluorophenyl)-1-(pyridin-3-ylsulfonyl)-1H-pyrrol-3-yl]-N-methylmethanamine monofumarate (TAK-438), a novel and potent potassium-competitive acid blocker for the treatment of acid-related diseases. | The Journal of pharmacology and experimental therapeutics 20101001 |