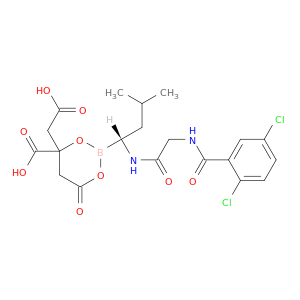
4-(Carboxymethyl)-2-((R)-1-(2-(2,5-dichlorobenzamido)acetamido)-3-methylbutyl)-6-oxo-1,3,2-dioxaborinane-4-carboxylic acid
| Title | Journal |
|---|---|
| The investigational proteasome inhibitor ixazomib for the treatment of multiple myeloma. | Future oncology (London, England) 20150101 |
| Ixazomib for the treatment of multiple myeloma. | Expert opinion on investigational drugs 20150101 |
| Linker for activation of T-cell family member2 (LAT2) a lipid raft adaptor protein for AKT signaling, is an early mediator of alkylphospholipid anti-leukemic activity. | Molecular & cellular proteomics : MCP 20121201 |
| Next-generation proteasome blockers promise safer cancer therapy. | Nature medicine 20120106 |
| Drugs: More shots on target. | Nature 20111214 |
| Antitumor activity of the investigational proteasome inhibitor MLN9708 in mouse models of B-cell and plasma cell malignancies. | Clinical cancer research : an official journal of the American Association for Cancer Research 20111201 |
| In vitro and in vivo selective antitumor activity of a novel orally bioavailable proteasome inhibitor MLN9708 against multiple myeloma cells. | Clinical cancer research : an official journal of the American Association for Cancer Research 20110815 |
| Chemical and biological evaluation of dipeptidyl boronic acid proteasome inhibitors for use in prodrugs and pro-soft drugs targeting solid tumors. | Journal of medicinal chemistry 20110714 |
| Evaluation of the proteasome inhibitor MLN9708 in preclinical models of human cancer. | Cancer research 20100301 |