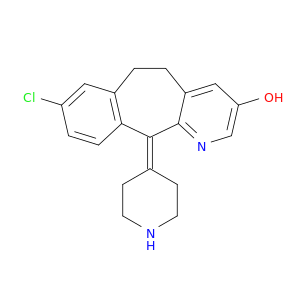
8-Chloro-11-(piperidin-4-ylidene)-6,11-dihydro-5H-benzo[5,6]cyclohepta[1,2-b]pyridin-3-ol
| Title | Journal |
|---|---|
| Prevalence of desloratadine poor metabolizer phenotype in healthy Jordanian males. | Biopharmaceutics & drug disposition 20120101 |
| Pharmacokinetic and safety profile of rupatadine when coadministered with azithromycin at steady-state levels: a randomized, open-label, two-way, crossover, Phase I study. | Clinical therapeutics 20080901 |
| Simultaneous determination of desloratadine and its active metabolite 3-hydroxydesloratadine in human plasma by LC/MS/MS and its application to pharmacokinetics and bioequivalence. | Journal of pharmaceutical and biomedical analysis 20071130 |
| Influence of food on the oral bioavailability of rupatadine tablets in healthy volunteers: a single-dose, randomized, open-label, two-way crossover study. | Clinical therapeutics 20070501 |
| Orthogonal extraction/chromatography and UPLC, two powerful new techniques for bioanalytical quantitation of desloratadine and 3-hydroxydesloratadine at 25 pg/mL. | Journal of pharmaceutical and biomedical analysis 20060224 |
| Pharmacokinetics/pharmacodynamics of desloratadine and fluoxetine in healthy volunteers. | Journal of clinical pharmacology 20041101 |
| Identification of human UDP-glucuronosyltransferase enzyme(s) responsible for the glucuronidation of 3-hydroxydesloratadine. | Biopharmaceutics & drug disposition 20040901 |
| Validation of a sensitive and automated 96-well solid-phase extraction liquid chromatography-tandem mass spectrometry method for the determination of desloratadine and 3-hydroxydesloratadine in human plasma. | Journal of chromatography. B, Analytical technologies in the biomedical and life sciences 20030725 |