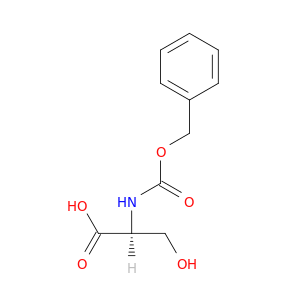
Z-Ser-OH
| Title | Journal |
|---|---|
| N-(2-oxo-3-oxetanyl)carbamic acid esters as N-acylethanolamine acid amidase inhibitors: synthesis and structure-activity and structure-property relationships. | Journal of medicinal chemistry 20120524 |
| Biochemical and mass spectrometric characterization of human N-acylethanolamine-hydrolyzing acid amidase inhibition. | PloS one 20120101 |
| From polyesters to polyamides via O-N acyl migration: an original multi-transfer reaction. | Macromolecular rapid communications 20110616 |
| Development of miracle medicines from sialic acids. | Proceedings of the Japan Academy. Series B, Physical and biological sciences 20110610 |
| Predicting binding to p-glycoprotein by flexible receptor docking. | PLoS computational biology 20110601 |
| Analyte separation by OMNiMIPs imprinted with multiple templates. | Biosensors & bioelectronics 20091115 |
| Transport and signaling via the amino acid binding site of the yeast Gap1 amino acid transceptor. | Nature chemical biology 20090101 |
| Synthesis and anticonvulsant evaluation of 6-amino-1,4-oxazepane-3,5-dione derivatives. | Archives of pharmacal research 20080701 |
| A short and efficient synthesis of L-5,5,5,5',5',5'-hexafluoroleucine from N-Cbz-L-serine. | Organic letters 20021128 |
| Beta-lactones as a new class of cysteine proteinase inhibitors: inhibition of hepatitis A virus 3C proteinase by N-Cbz-serine beta-lactone. | Organic letters 19990909 |