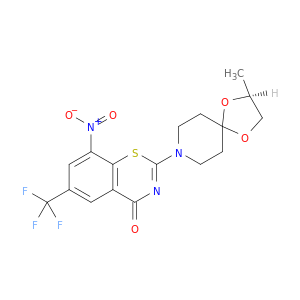
4H-1,3-Benzothiazin-4-one, 2-[(2S)-2-methyl-1,4-dioxa-8-azaspiro[4.5]dec-8-yl]-8-nitro-6-(trifluoromethyl)-
| Title | Journal |
|---|---|
| Synthesis and structure-activity relationships evaluation of benzothiazinone derivatives as potential anti-tubercular agents. | Bioorganic & medicinal chemistry letters 20130901 |
| Identification of antitubercular benzothiazinone compounds by ligand-based design. | Journal of medicinal chemistry 20120913 |
| Tuberculosis: the drug development pipeline at a glance. | European journal of medicinal chemistry 20120501 |
| Benzothiazinones kill Mycobacterium tuberculosis by blocking arabinan synthesis. | Science (New York, N.Y.) 20090508 |