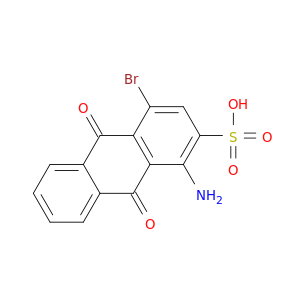
Bromaminic acid
| Title | Journal |
|---|---|
| Convergent synthesis of the potent P2Y receptor antagonist MG 50-3-1 based on a regioselective Ullmann coupling reaction. | Molecules (Basel, Switzerland) 20120305 |
| Immobilized laccase on a new cryogel carrier and kinetics of two anthraquinone derivatives oxidation. | Applied biochemistry and biotechnology 20111201 |
| Structural insights into the inhibition of cytosolic 5'-nucleotidase II (cN-II) by ribonucleoside 5'-monophosphate analogues. | PLoS computational biology 20111201 |
| Biodegradation of bromoamine acid using combined airlift loop reactor and biological activated carbon. | Bioresource technology 20110301 |
| Synthesis of alkyl- and aryl-amino-substituted anthraquinone derivatives by microwave-assisted copper(0)-catalyzed Ullmann coupling reactions. | Nature protocols 20100501 |
| Structure-activity relationships of anthraquinone derivatives derived from bromaminic acid as inhibitors of ectonucleoside triphosphate diphosphohydrolases (E-NTPDases). | Purinergic signalling 20090301 |
| Influence of Ecto-nucleoside triphosphate diphosphohydrolase activity on Trypanosoma cruzi infectivity and virulence. | PLoS neglected tropical diseases 20090301 |
| [Degradation characteristics of bromoamine acid by Sphingomonas sp. FL]. | Huan jing ke xue= Huanjing kexue 20080901 |
| The new incorporation bio-treatment technology of bromoamine acid and azo dyes wastewaters under high-salt conditions. | Biodegradation 20080201 |
| Combinatorial synthesis of anilinoanthraquinone derivatives and evaluation as non-nucleotide-derived P2Y2 receptor antagonists. | Bioorganic & medicinal chemistry letters 20080101 |
| Rapid and efficient microwave-assisted copper(0)-catalyzed ullmann coupling reaction: general access to anilinoanthraquinone derivatives. | Organic letters 20070329 |