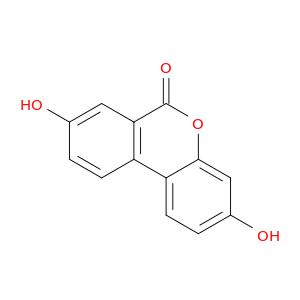
3,8-Dihydroxy-6H-benzo[c]chromen-6-one
| Title | Journal |
|---|---|
| Phase II Conjugates of Urolithins Isolated from Human Urine and Potential Role of β-Glucuronidases in Their Disposition. | Drug metabolism and disposition: the biological fate of chemicals 20170601 |
| In vitro antioxidant and antiproliferative effects of ellagic acid and its colonic metabolite, urolithins, on human bladder cancer T24 cells. | Food and chemical toxicology : an international journal published for the British Industrial Biological Research Association 20130901 |
| Biological significance of urolithins, the gut microbial ellagic Acid-derived metabolites: the evidence so far. | Evidence-based complementary and alternative medicine : eCAM 20130101 |
| Intestinal ellagitannin metabolites ameliorate cytokine-induced inflammation and associated molecular markers in human colon fibroblasts. | Journal of agricultural and food chemistry 20120912 |
| Ellagic acid and polyhydroxylated urolithins are potent catalytic inhibitors of human topoisomerase II: an in vitro study. | Journal of agricultural and food chemistry 20120912 |
| Ellagitannin metabolites, urolithin A glucuronide and its aglycone urolithin A, ameliorate TNF-α-induced inflammation and associated molecular markers in human aortic endothelial cells. | Molecular nutrition & food research 20120501 |
| Metabolism of oak leaf ellagitannins and urolithin production in beef cattle. | Journal of agricultural and food chemistry 20120328 |
| Urolithin as a converging scaffold linking ellagic acid and coumarin analogues: design of potent protein kinase CK2 inhibitors. | ChemMedChem 20111209 |
| In vivo anti-inflammatory and antioxidant properties of ellagitannin metabolite urolithin A. | Bioorganic & medicinal chemistry letters 20111001 |
| Metabolites of the ellagitannin geraniin and their antioxidant activities. | Planta medica 20110701 |
| Anti-inflammatory properties of a pomegranate extract and its metabolite urolithin-A in a colitis rat model and the effect of colon inflammation on phenolic metabolism. | The Journal of nutritional biochemistry 20100801 |
| Effects of fruit ellagitannin extracts, ellagic acid, and their colonic metabolite, urolithin A, on Wnt signaling. | Journal of agricultural and food chemistry 20100414 |
| Occurrence of urolithins, gut microbiota ellagic acid metabolites and proliferation markers expression response in the human prostate gland upon consumption of walnuts and pomegranate juice. | Molecular nutrition & food research 20100301 |
| Pomegranate ellagitannin-derived compounds exhibit antiproliferative and antiaromatase activity in breast cancer cells in vitro. | Cancer prevention research (Philadelphia, Pa.) 20100101 |
| Effects of pomegranate chemical constituents/intestinal microbial metabolites on CYP1B1 in 22Rv1 prostate cancer cells. | Journal of agricultural and food chemistry 20091125 |
| Urolithins, intestinal microbial metabolites of Pomegranate ellagitannins, exhibit potent antioxidant activity in a cell-based assay. | Journal of agricultural and food chemistry 20091111 |
| Interaction of polyphenolic metabolites with human serum albumin: a circular dichroism study. | Chemical & pharmaceutical bulletin 20090901 |
| A concise synthesis of glucuronide metabolites of urolithin-B, resveratrol, and hydroxytyrosol. | Carbohydrate research 20090727 |
| Dissimilar in vitro and in vivo effects of ellagic acid and its microbiota-derived metabolites, urolithins, on the cytochrome P450 1A1. | Journal of agricultural and food chemistry 20090624 |
| Gene expression, cell cycle arrest and MAPK signalling regulation in Caco-2 cells exposed to ellagic acid and its metabolites, urolithins. | Molecular nutrition & food research 20090601 |
| Pomegranate juice and extracts provide similar levels of plasma and urinary ellagitannin metabolites in human subjects. | Journal of medicinal food 20080601 |
| Pomegranate ellagitannin-derived metabolites inhibit prostate cancer growth and localize to the mouse prostate gland. | Journal of agricultural and food chemistry 20070919 |
| Urolithins, ellagic acid-derived metabolites produced by human colonic microflora, exhibit estrogenic and antiestrogenic activities. | Journal of agricultural and food chemistry 20060308 |
| Identification of urolithin a as a metabolite produced by human colon microflora from ellagic acid and related compounds. | Journal of agricultural and food chemistry 20050713 |
| Metabolism of antioxidant and chemopreventive ellagitannins from strawberries, raspberries, walnuts, and oak-aged wine in humans: identification of biomarkers and individual variability. | Journal of agricultural and food chemistry 20050126 |