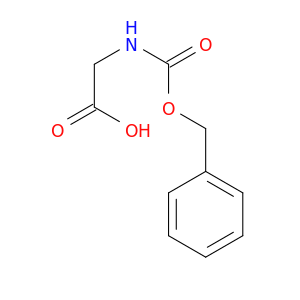
Z-Gly-OH
| Title | Journal |
|---|---|
| Imidazolopiperazines: lead optimization of the second-generation antimalarial agents. | Journal of medicinal chemistry 20120510 |
| Controlled functionalization of carbon nanotubes by a solvent-free multicomponent approach. | ACS nano 20101228 |
| Substituted hippurates and hippurate analogs as substrates and inhibitors of peptidylglycine alpha-hydroxylating monooxygenase (PHM). | Bioorganic & medicinal chemistry 20081201 |
| Duality of mechanism in the tetramethylfluoroformamidinium hexafluorophosphate-mediated synthesis of N-benzyloxycarbonylamino acid fluorides. | The Journal of organic chemistry 20010824 |