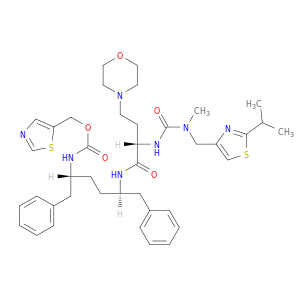
1,3-thiazol-5-ylmethyl N-[(2R,5R)-5-[(2S)-2-{[methyl({[2-(propan-2-yl)-1,3-thiazol-4-yl]methyl})carbamoyl]amino}-4-(morpholin-4-yl)butanamido]-1,6-diphenylhexan-2-yl]carbamate
| Title | Journal |
|---|---|
| Pharmacokinetic enhancers in HIV therapeutics. | Clinical pharmacokinetics 20141001 |
| Cobicistat boosts the intestinal absorption of transport substrates, including HIV protease inhibitors and GS-7340, in vitro. | Antimicrobial agents and chemotherapy 20121001 |
| Effect of cobicistat on glomerular filtration rate in subjects with normal and impaired renal function. | Journal of acquired immune deficiency syndromes (1999) 20120901 |
| Combinational therapies for HIV: a focus on EVG/COBI/FTC/TDF. | Expert opinion on pharmacotherapy 20120901 |
| [A single tablet against HIV: new combination preparation improves therapy]. | Deutsche medizinische Wochenschrift (1946) 20120801 |
| Co-formulated elvitegravir, cobicistat, emtricitabine, and tenofovir disoproxil fumarate versus ritonavir-boosted atazanavir plus co-formulated emtricitabine and tenofovir disoproxil fumarate for initial treatment of HIV-1 infection: a randomised, double-blind, phase 3, non-inferiority trial. | Lancet (London, England) 20120630 |
| Co-formulated elvitegravir, cobicistat, emtricitabine, and tenofovir versus co-formulated efavirenz, emtricitabine, and tenofovir for initial treatment of HIV-1 infection: a randomised, double-blind, phase 3 trial, analysis of results after 48 weeks. | Lancet (London, England) 20120630 |
| Phase 2 study of cobicistat versus ritonavir each with once-daily atazanavir and fixed-dose emtricitabine/tenofovir df in the initial treatment of HIV infection. | AIDS (London, England) 20110924 |
| Randomized, phase 2 evaluation of two single-tablet regimens elvitegravir/cobicistat/emtricitabine/tenofovir disoproxil fumarate versus efavirenz/emtricitabine/tenofovir disoproxil fumarate for the initial treatment of HIV infection. | AIDS (London, England) 20110327 |
| Pharmacokinetics and bioavailability of an integrase and novel pharmacoenhancer-containing single-tablet fixed-dose combination regimen for the treatment of HIV. | Journal of acquired immune deficiency syndromes (1999) 20101101 |
| Cobicistat (GS-9350): A Potent and Selective Inhibitor of Human CYP3A as a Novel Pharmacoenhancer. | ACS medicinal chemistry letters 20100812 |
| Pharmacokinetics and pharmacodynamics of GS-9350: a novel pharmacokinetic enhancer without anti-HIV activity. | Clinical pharmacology and therapeutics 20100301 |