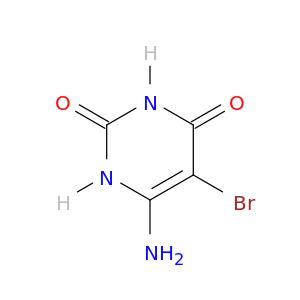
5-BROMO-6-AMINOURACIL
| Title | Journal |
|---|---|
| Fluorophosphonylated nucleoside derivatives as new series of thymidine phosphorylase multisubstrate inhibitors. | Journal of medicinal chemistry 20120322 |
| The role of phosphate in the action of thymidine phosphorylase inhibitors: Implications for the catalytic mechanism. | Bioorganic & medicinal chemistry letters 20100301 |
| Identification of aspartic acid-203 in human thymidine phosphorylase as an important residue for both catalysis and non-competitive inhibition by the small molecule 'crystallization chaperone' 5'-O-tritylinosine (KIN59). | Biochemical pharmacology 20090801 |
| Proteomics identifies thymidine phosphorylase as a key regulator of the angiogenic potential of colony-forming units and endothelial progenitor cell cultures. | Circulation research 20090102 |
| Xanthine oxidase-activated prodrugs of thymidine phosphorylase inhibitors. | European journal of medicinal chemistry 20080601 |
| Aminoimidazolylmethyluracil analogues as potent inhibitors of thymidine phosphorylase and their bioreductive nitroimidazolyl prodrugs. | Journal of medicinal chemistry 20050127 |
| Synthesis and enzymatic evaluation of xanthine oxidase-activated prodrugs based on inhibitors of thymidine phosphorylase. | Bioorganic & medicinal chemistry letters 20041101 |
| Identification of a novel class of inhibitor of human and Escherichia coli thymidine phosphorylase by in silico screening. | Bioorganic & medicinal chemistry letters 20031103 |
| Potential tumor-selective nitroimidazolylmethyluracil prodrug derivatives: inhibitors of the angiogenic enzyme thymidine phosphorylase. | Journal of medicinal chemistry 20030116 |